
Give the name of the following compound:
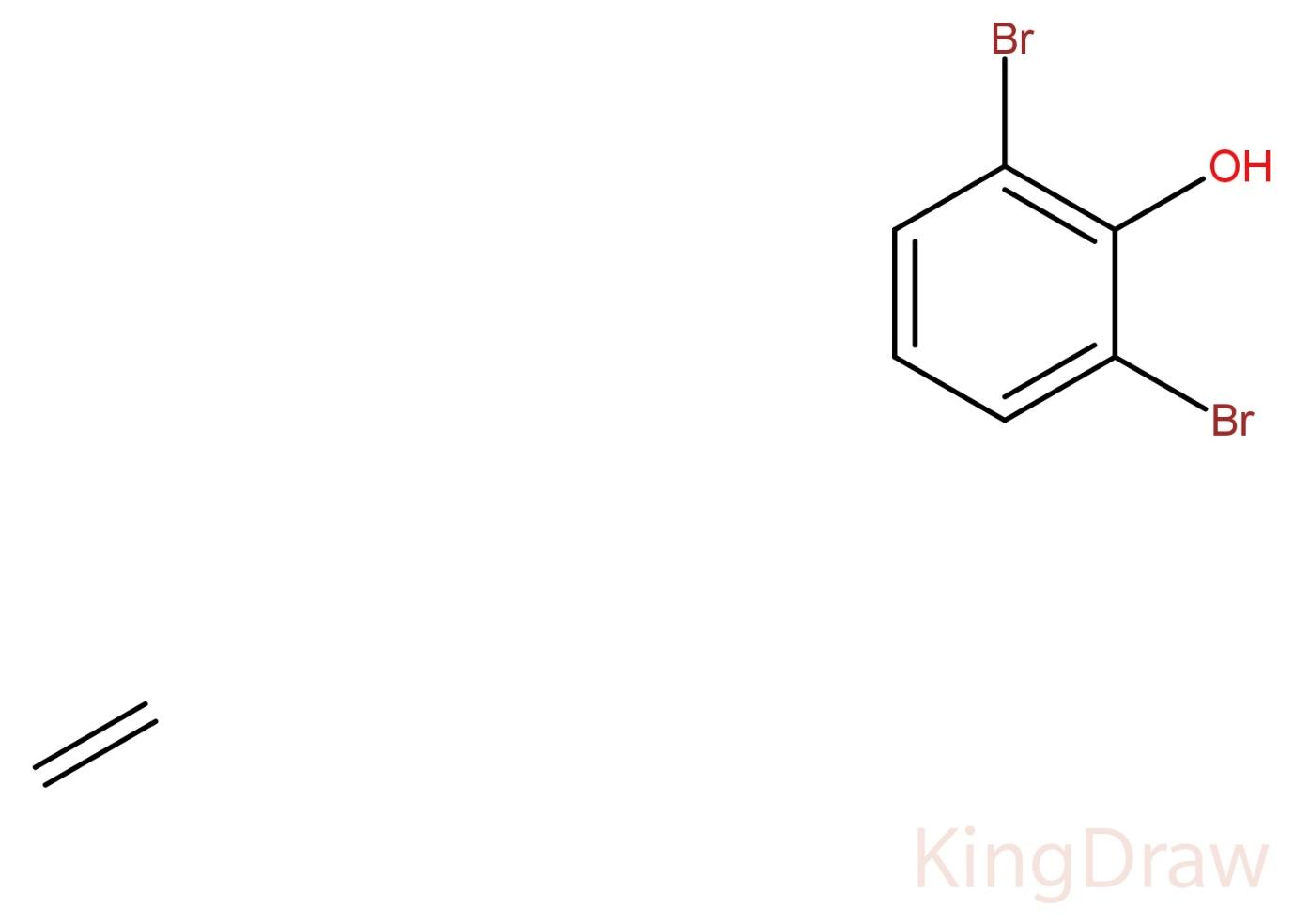
(A) 1,3 – dibromophenol
(B) 1,2 – dibromophenol
(C) 2,6 – dibromophenol
(D) 1,6 – dibromophenol

Answer
605.7k+ views
Hint: Phenol is nothing but a hydroxyl group (-OH) attached to a benzene ring. When the phenol derivative is numbered according to the IUPAC norms, the carbon to which the hydroxyl group is attached is numbered as 1.
Complete step by step answer:
When a compound is numbered by the IUPAC standards, the functional group with the highest priority gives its suffix to the name of the molecule. The priority order of IUPAC in descending order is: Carboxylic acid, sulfonic acid, ester, acid halide, amide, nitrile, aldehyde, ketone, alcohol, amine. For alkenes and alkynes, the suffix is -ene and -yne respectively.
In the above given compound, the carbonyl carbon to which the hydroxyl group is attached will be numbered one (1). Then, the compound is symmetric from both the sides, so we can number the other carbons from either side. So, the first bromine will be numbered 2. Then after 3 ring carbons, the other bromine will be numbered 6. Hence, the numbers designated to both the bromines are 2 and 6. As the compound has two bromines, a prefix ‘di’ will be used in its IUPAC naming.
Therefore, the IUPAC name of the given compound will be 2,6 – dibromophenol.
Hence, the correct answer is (C) 2,6 – dibromophenol.
Note: Make sure to remember that while numbering the carbons, if the priority order of any two functional groups is the same, then the carbons are numbered according to alphabetical preference.
Complete step by step answer:
When a compound is numbered by the IUPAC standards, the functional group with the highest priority gives its suffix to the name of the molecule. The priority order of IUPAC in descending order is: Carboxylic acid, sulfonic acid, ester, acid halide, amide, nitrile, aldehyde, ketone, alcohol, amine. For alkenes and alkynes, the suffix is -ene and -yne respectively.
In the above given compound, the carbonyl carbon to which the hydroxyl group is attached will be numbered one (1). Then, the compound is symmetric from both the sides, so we can number the other carbons from either side. So, the first bromine will be numbered 2. Then after 3 ring carbons, the other bromine will be numbered 6. Hence, the numbers designated to both the bromines are 2 and 6. As the compound has two bromines, a prefix ‘di’ will be used in its IUPAC naming.
Therefore, the IUPAC name of the given compound will be 2,6 – dibromophenol.
Hence, the correct answer is (C) 2,6 – dibromophenol.
Note: Make sure to remember that while numbering the carbons, if the priority order of any two functional groups is the same, then the carbons are numbered according to alphabetical preference.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




