
Glucose gives solver mirror with Tollen reagent, it shows the presence of:
A. A kenotic group
B. An aldehydic group
C. An alcoholic group
D. An acidic group
Answer
563.4k+ views
Hint: To determine the answer we should know the structure of the glucose and what is Tollen reagent. Glucose is a hexose sugar having six carbon and aldehydic functional groups. By the reaction of glucose with Tollens reagent a silver mirror of elemental silver forms. Tollen’s reagent gives a positive test for alpha-hydroxy ketones.
Complete solution:
Tollen’s reagent is formed by silver nitrate, sodium hydroxide which forms silver oxide and sodium nitrate. On adding ammonia to this solution a diamminesilver (I) complex forms which is known as Tollen’s reagent.
The reaction of glucose with Tollens reagent (diamminesilver (I) complex) is as follows:
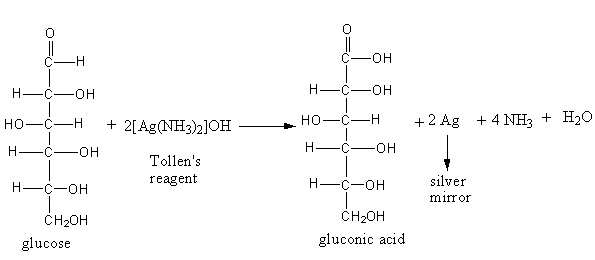
When glucose reacts with Tollen’s reagent, the elemental silver precipitates which get deposited on the wall of the test tube. The precipitate is known as a silver mirror. Tollen’s reagent gives a positive test for the compounds having an aldehydic functional group.So, glucose gives solver mirror with Tollen reagent, it shows the presence of an aldehydic group.
Thus, the correct option is (B).
Note:Tollen’s reagent is used to determine the presence of the carbonyl group in chemical compounds. The carbonyl group contains aldehyde and ketone. Tollen’s reagent test is used to differentiate the aldehydes and ketones. The chemical compound having a ketone functional group gives the oxime and hydroxylamine with Tollen’s reagent whereas the chemical compound having an aldehyde functional group gives the silver mirror. The glucose is a reducing sugar because it has a free aldehyde group that can react. So, even the disaccharides have an aldehyde functional group they do not react with Tollen’s reagent.
Complete solution:
Tollen’s reagent is formed by silver nitrate, sodium hydroxide which forms silver oxide and sodium nitrate. On adding ammonia to this solution a diamminesilver (I) complex forms which is known as Tollen’s reagent.
The reaction of glucose with Tollens reagent (diamminesilver (I) complex) is as follows:

When glucose reacts with Tollen’s reagent, the elemental silver precipitates which get deposited on the wall of the test tube. The precipitate is known as a silver mirror. Tollen’s reagent gives a positive test for the compounds having an aldehydic functional group.So, glucose gives solver mirror with Tollen reagent, it shows the presence of an aldehydic group.
Thus, the correct option is (B).
Note:Tollen’s reagent is used to determine the presence of the carbonyl group in chemical compounds. The carbonyl group contains aldehyde and ketone. Tollen’s reagent test is used to differentiate the aldehydes and ketones. The chemical compound having a ketone functional group gives the oxime and hydroxylamine with Tollen’s reagent whereas the chemical compound having an aldehyde functional group gives the silver mirror. The glucose is a reducing sugar because it has a free aldehyde group that can react. So, even the disaccharides have an aldehyde functional group they do not react with Tollen’s reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




