
What happens when chlorobenzene is subjected to hydrolysis?
Answer
539.7k+ views
Hint:Chlorobenzene is an aromatic compound in which a chlorine atom is present on the benzene ring when it is hydrolyzed, it is reacted with water. Under specific conditions, the chlorine on the benzene ring will be replaced with a hydroxyl group.
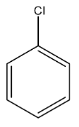
Complete step-by-step answer:Chlorobenzene is an aromatic compound in which a chlorine atom is present on the benzene ring. The formula of the chlorobenzene is ${{C}_{6}}{{H}_{5}}Cl$. The chlorobenzene is a liquid and it must be handled with care because it is a flammable liquid. The structure of chlorobenzene is given below:
When a compound is hydrolyzed, this means that the compound is treated with water. When the chlorobenzene is treated with water or hydrolyzed under normal conditions, then no changes or no reaction takes place.
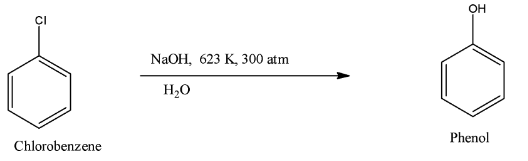
For the reaction to occur, the chlorobenzene is treated with water along with a base like sodium hydroxide (NaOH) and under specific temperature and pressure. The temperature must be kept at 623 K and the pressure must be 300 atm.
So, when these conditions are satisfied then the chlorine atom on the benzene ring is replaced with the hydroxyl group. Therefore, the compound formed is phenol. The formula of phenol is ${{C}_{6}}{{H}_{5}}OH$. The reaction is given below:
Another name of phenol is carbolic acid. Phenol is a solid which has a white color and it is volatile.
Note:The chlorobenzene undergoes many other reactions like nitration, sulfonation, etc. In nitration, there will be the addition of the nitro group on ortho and para position and in sulfonation, there will be the addition of $S{{O}_{3}}H$ on ortho and para position.
Complete step-by-step answer:Chlorobenzene is an aromatic compound in which a chlorine atom is present on the benzene ring. The formula of the chlorobenzene is ${{C}_{6}}{{H}_{5}}Cl$. The chlorobenzene is a liquid and it must be handled with care because it is a flammable liquid. The structure of chlorobenzene is given below:

When a compound is hydrolyzed, this means that the compound is treated with water. When the chlorobenzene is treated with water or hydrolyzed under normal conditions, then no changes or no reaction takes place.
For the reaction to occur, the chlorobenzene is treated with water along with a base like sodium hydroxide (NaOH) and under specific temperature and pressure. The temperature must be kept at 623 K and the pressure must be 300 atm.
So, when these conditions are satisfied then the chlorine atom on the benzene ring is replaced with the hydroxyl group. Therefore, the compound formed is phenol. The formula of phenol is ${{C}_{6}}{{H}_{5}}OH$. The reaction is given below:

Another name of phenol is carbolic acid. Phenol is a solid which has a white color and it is volatile.
Note:The chlorobenzene undergoes many other reactions like nitration, sulfonation, etc. In nitration, there will be the addition of the nitro group on ortho and para position and in sulfonation, there will be the addition of $S{{O}_{3}}H$ on ortho and para position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




