
How can I graph Charles law?
Answer
562.8k+ views
Hint: The gas law which contains terms like temperature (T), pressure (P), volume (V) gives a relation between them, which is PV = nRT. This is also known as the ideal gas law.
Complete step by step answer:
We know that the expression for the ideal gas law is mentioned below:
PV = nRT
Where, P is the pressure of the gas
V is the volume of the gas
N is the number of moles of gas
R is the universal gas constant
T is the temperature of the gas
Charles law: according to this law the volume of an ideal gas changes with the change in the absolute temperature, when the pressure of the system is constant throughout the experiment. It is the ratio of volume of temperature where the pressure is always constant.
\[\dfrac{{{V}_{1}}}{{{T}_{1}}}=\dfrac{{{V}_{2}}}{{{T}_{2}}}\]
Pressure and number of moles are constant.
The graph of Charles law is as follows:
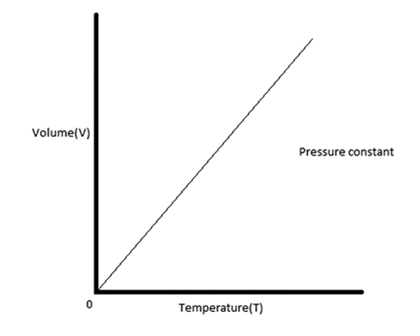
Additional Information .
- Boyle’s law: According to this law at the constant temperature, the pressure applied to an ideal gas is inversely proportional to the volume of the system. This means that the product of pressure and volume is constant.
\[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\]
Temperature is constant.
- Avogadro’s law: according to this law at constant pressure and absolute temperature, the number of moles is directly proportional to the volume of the gas. This means that the ratio of volume and number of moles is constant.
\[\dfrac{{{V}_{1}}}{{{n}_{1}}}=\dfrac{{{V}_{2}}}{{{n}_{2}}}\]
Note: If we combine Boyles and Charles law a new equation is formed:
\[\dfrac{{{P}_{1}}{{V}_{1}}}{{{T}_{1}}}=\dfrac{{{P}_{2}}{{V}_{2}}}{{{T}_{2}}}\]
In this equation pressure, volume and temperature all are variables and also the product of pressure and volume in the reaction changes proportionally with the absolute temperature.
Complete step by step answer:
We know that the expression for the ideal gas law is mentioned below:
PV = nRT
Where, P is the pressure of the gas
V is the volume of the gas
N is the number of moles of gas
R is the universal gas constant
T is the temperature of the gas
Charles law: according to this law the volume of an ideal gas changes with the change in the absolute temperature, when the pressure of the system is constant throughout the experiment. It is the ratio of volume of temperature where the pressure is always constant.
\[\dfrac{{{V}_{1}}}{{{T}_{1}}}=\dfrac{{{V}_{2}}}{{{T}_{2}}}\]
Pressure and number of moles are constant.
The graph of Charles law is as follows:

Additional Information .
- Boyle’s law: According to this law at the constant temperature, the pressure applied to an ideal gas is inversely proportional to the volume of the system. This means that the product of pressure and volume is constant.
\[{{P}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\]
Temperature is constant.
- Avogadro’s law: according to this law at constant pressure and absolute temperature, the number of moles is directly proportional to the volume of the gas. This means that the ratio of volume and number of moles is constant.
\[\dfrac{{{V}_{1}}}{{{n}_{1}}}=\dfrac{{{V}_{2}}}{{{n}_{2}}}\]
Note: If we combine Boyles and Charles law a new equation is formed:
\[\dfrac{{{P}_{1}}{{V}_{1}}}{{{T}_{1}}}=\dfrac{{{P}_{2}}{{V}_{2}}}{{{T}_{2}}}\]
In this equation pressure, volume and temperature all are variables and also the product of pressure and volume in the reaction changes proportionally with the absolute temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




