
How would you ethyl ethanoate?
Answer
556.5k+ views
Hint: We know that how ethyl ethanoate or ethyl acetate is an ester and general method of ester preparation can be given bY the reversible reaction: \[Acid\text{ }+\text{ }Alcohol\text{ }\to \text{ }Ester\text{ }+\text{ }Water\] and conc. sulphuric acid is used to catalyze it.
Complete step-by-step answer:The ethyl ethanoate is in general prepared by gently giving heat to a mixture of ethanoic acid, ethanol, and conc. sulphuric acid which is either in a water bath else in an electric heater. This reaction is also called the esterification. The chemical
reaction for preparation of ethyl ethanoate is given as:
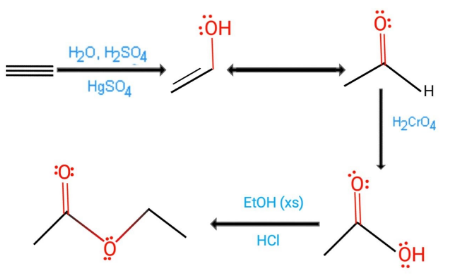
1.A simple acidic catalyze of hydration which is except to get updated by adding a mercury sulfate for decrease in reactivity of alkyne in order to acquire hydration reaction which in comparison to alkene.
2.The keto-enol tautomerization in an acid which is eons in order to form aldehyde.
3.Oxidation to the carboxylic acid through chromic acid.
4.Esterification of a carboxylic acid by reacting with excess ethanol in acidic conditions.
The esters form during above reaction are distilled off as soon as possible as well as cool in the separate beaker by the process of condensation and since above reaction that is esterification reaction which is reversible here ester form is removed by the distillation from the reaction mixture. By the process of Esterification we commonly use products like biodiesel, pharmaceutical, solvent of paint, adhesive, pesticides.
Note:Note that the Ethyl ethanoate is an organic compound which is mainly used as a solvent in some different chemical reactions. The ethyl ethanoate is a highly flammable as well as it’s generally found in kinds of alcoholic drinks such as wines, etc. Also it is a colorless, sweet smelling ester.
Complete step-by-step answer:The ethyl ethanoate is in general prepared by gently giving heat to a mixture of ethanoic acid, ethanol, and conc. sulphuric acid which is either in a water bath else in an electric heater. This reaction is also called the esterification. The chemical
reaction for preparation of ethyl ethanoate is given as:

1.A simple acidic catalyze of hydration which is except to get updated by adding a mercury sulfate for decrease in reactivity of alkyne in order to acquire hydration reaction which in comparison to alkene.
2.The keto-enol tautomerization in an acid which is eons in order to form aldehyde.
3.Oxidation to the carboxylic acid through chromic acid.
4.Esterification of a carboxylic acid by reacting with excess ethanol in acidic conditions.
The esters form during above reaction are distilled off as soon as possible as well as cool in the separate beaker by the process of condensation and since above reaction that is esterification reaction which is reversible here ester form is removed by the distillation from the reaction mixture. By the process of Esterification we commonly use products like biodiesel, pharmaceutical, solvent of paint, adhesive, pesticides.
Note:Note that the Ethyl ethanoate is an organic compound which is mainly used as a solvent in some different chemical reactions. The ethyl ethanoate is a highly flammable as well as it’s generally found in kinds of alcoholic drinks such as wines, etc. Also it is a colorless, sweet smelling ester.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




