
Hybridization of sulfur in ${{H}_{2}}S{{O}_{4}}$ is:
(a)- $sp$
(b)- $s{{p}^{2}}$
(c)- $s{{p}^{3}}$
(d)- $s{{p}^{3}}{{d}^{2}}$
Answer
585.3k+ views
Hint: The hybridization can be calculated with the number of valence electrons of the central atom, number of monovalent atoms/ groups surrounding the central atom, charge on the cation, and charge on the anion.
Complete step by step answer:
Hybridization is defined as the mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that redistribution of energy takes place between them which results in the formation of new orbitals with similar energies and similar shapes. The orbitals thus formed are known as hybrid orbitals.
In ${{H}_{2}}S{{O}_{4}}$ the central atom is sulfur and it is surrounded by four oxygen atoms and two hydrogen atoms.
For calculating the number of hybrid orbital or hybridization of the central atom we can use the formula:
$X=\dfrac{1}{2}\left[ \begin{align}
& \{\text{no}\text{. of valence electrons of central atom }\!\!\}\!\!\text{ + }\!\!\{\!\!\text{ no}\text{. of monovalent atoms }\!\!\}\!\!\text{ } \\
& \text{ - }\!\!\{\!\!\text{ charge on cation }\!\!\}\!\!\text{ + }\!\!\{\!\!\text{ charge on the anion }\!\!\}\!\!\text{ } \\
\end{align} \right]$
$X=\dfrac{1}{2}\left[ VE+MA-c+a \right]$
So, in ${{H}_{2}}S{{O}_{4}}$the central atom sulfur has 6 valence electrons.
There are two monovalent atoms present in ${{H}_{2}}S{{O}_{4}}$. Oxygen is a divalent atom.
There is no cationic charge in ${{H}_{2}}S{{O}_{4}}$.
There is no anionic charge in ${{H}_{2}}S{{O}_{4}}$
So, by putting all these in the equation, we get
$X=\dfrac{1}{2}\left[ 6+2-0+0 \right]=\dfrac{8}{2}=4$
The value of X is 4, therefore, the hybridization is$s{{p}^{3}}$.
The structure of sulfuric acid (${{H}_{2}}S{{O}_{4}}$) is given below:
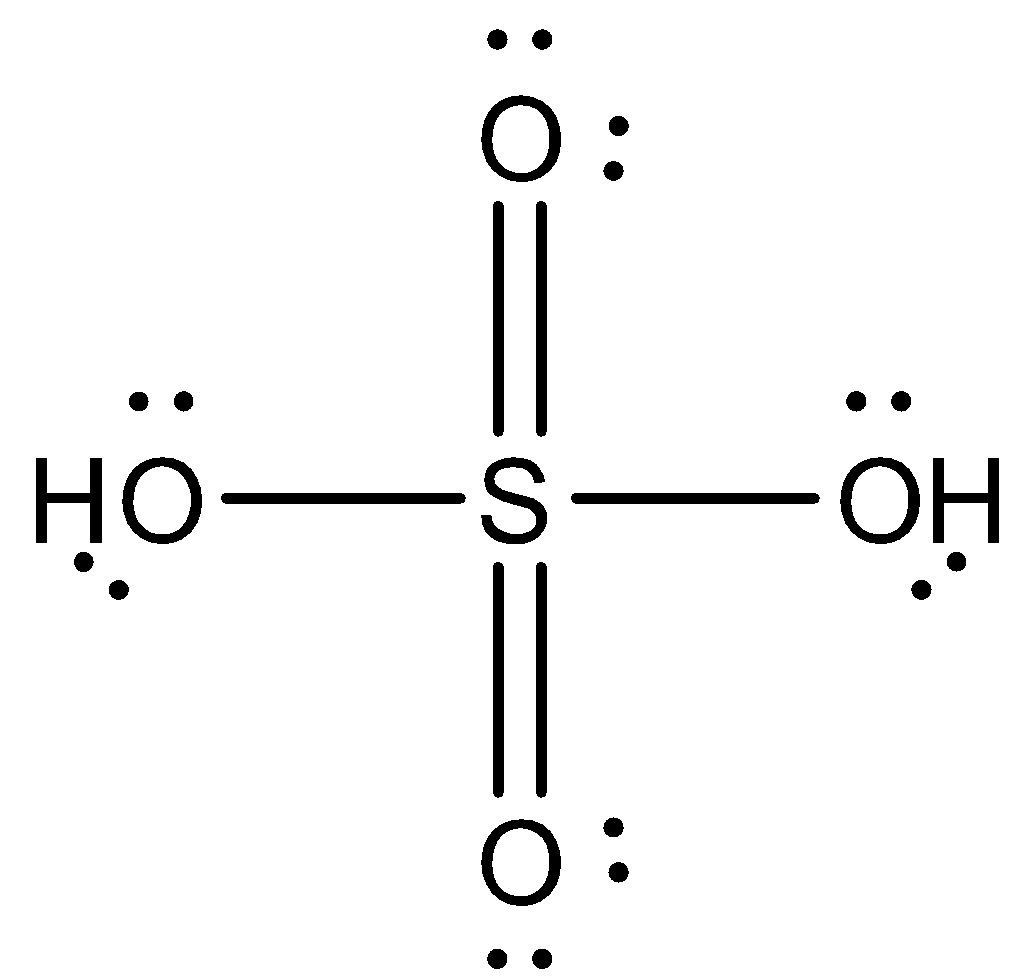
Because of $s{{p}^{3}}$hybridization, the structure of sulfuric acid (${{H}_{2}}S{{O}_{4}}$) is tetrahedral.
Therefore the correct answer is an option (c)- $s{{p}^{3}}$
Note: Only monovalent atoms can be considered. For divalent ion, MA = 0. By calculating the hybridization the structure can be predicted, but due lone pair the structure will get changed. So for predicting the shape, lone pairs should be considered.
Complete step by step answer:
Hybridization is defined as the mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that redistribution of energy takes place between them which results in the formation of new orbitals with similar energies and similar shapes. The orbitals thus formed are known as hybrid orbitals.
In ${{H}_{2}}S{{O}_{4}}$ the central atom is sulfur and it is surrounded by four oxygen atoms and two hydrogen atoms.
For calculating the number of hybrid orbital or hybridization of the central atom we can use the formula:
$X=\dfrac{1}{2}\left[ \begin{align}
& \{\text{no}\text{. of valence electrons of central atom }\!\!\}\!\!\text{ + }\!\!\{\!\!\text{ no}\text{. of monovalent atoms }\!\!\}\!\!\text{ } \\
& \text{ - }\!\!\{\!\!\text{ charge on cation }\!\!\}\!\!\text{ + }\!\!\{\!\!\text{ charge on the anion }\!\!\}\!\!\text{ } \\
\end{align} \right]$
$X=\dfrac{1}{2}\left[ VE+MA-c+a \right]$
So, in ${{H}_{2}}S{{O}_{4}}$the central atom sulfur has 6 valence electrons.
There are two monovalent atoms present in ${{H}_{2}}S{{O}_{4}}$. Oxygen is a divalent atom.
There is no cationic charge in ${{H}_{2}}S{{O}_{4}}$.
There is no anionic charge in ${{H}_{2}}S{{O}_{4}}$
So, by putting all these in the equation, we get
$X=\dfrac{1}{2}\left[ 6+2-0+0 \right]=\dfrac{8}{2}=4$
The value of X is 4, therefore, the hybridization is$s{{p}^{3}}$.
The structure of sulfuric acid (${{H}_{2}}S{{O}_{4}}$) is given below:

Because of $s{{p}^{3}}$hybridization, the structure of sulfuric acid (${{H}_{2}}S{{O}_{4}}$) is tetrahedral.
Therefore the correct answer is an option (c)- $s{{p}^{3}}$
Note: Only monovalent atoms can be considered. For divalent ion, MA = 0. By calculating the hybridization the structure can be predicted, but due lone pair the structure will get changed. So for predicting the shape, lone pairs should be considered.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




