
Identify A-F in the given flow chart.
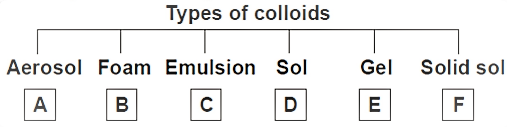
A) A: Jelly B: Shaving Cream C: Milk D: Fog E: Cheese F: Gemstones
B) A: Milk of Magnesia B: Shaving Cream C: Mud D: Cloud E: Butter F: Gemstones
C) A: Cloud B: Shaving Cream C: Jelly D: Fog E: Rubber F: Gemstones
D) A: Fog B: Shaving Cream C: Milk D: Mud E: Jelly F: Gemstones

Answer
567.9k+ views
Hint: Colloids are very small particles of any substance, dispersed throughout other substances. They do not settle when left undisturbed. They come under heterogeneous mixtures. Colloidal particles will have enormous surface area per unit mass due to their small size.
Complete Solution:
Before moving onto colloids, let’s first get some idea about what a mixture is. Mixture is the physical combination of two or more substances without undergoing any chemical changes. Mixtures are the blending of chemical substances such as elements and compounds.
There are two types of mixtures:
- Homogeneous mixture
- Heterogeneous mixture
Colloids are very small particles of any substance, dispersed throughout other substances. They do not settle when left undisturbed. They come under heterogeneous mixtures.
Colloids are classified based on the physical states of components: This type of classification is based on the physical state of the dispersed phase and dispersion medium. Based on whether the dispersed phase and dispersion medium is solid, liquid or gases, we can classify the colloids into eight types.
-Now, let us discuss the options that are given to us.
-Fog is an aerosol. Fogs are formed in the atmosphere where the gas and liquid mix as dispersion medium and dispersed phase respectively.
-Shaving cream is a foam.
-Milk is Emulsion.
-Mud is a Sol.
-Jelly is a gel.
-Gemstones are Solid sols.
So, the correct answer is “Option D”.
Note: A gas mixed with other gas forms a homogeneous mixture; hence this system is not a colloidal system. The hydrophilic sols are more stable than hydrophobic sols because the stability of hydrophobic sols is due to charge only and hydrophilic sols are stable due to charge and solvation.
Complete Solution:
Before moving onto colloids, let’s first get some idea about what a mixture is. Mixture is the physical combination of two or more substances without undergoing any chemical changes. Mixtures are the blending of chemical substances such as elements and compounds.
There are two types of mixtures:
- Homogeneous mixture
- Heterogeneous mixture
Colloids are very small particles of any substance, dispersed throughout other substances. They do not settle when left undisturbed. They come under heterogeneous mixtures.
Colloids are classified based on the physical states of components: This type of classification is based on the physical state of the dispersed phase and dispersion medium. Based on whether the dispersed phase and dispersion medium is solid, liquid or gases, we can classify the colloids into eight types.
| Dispersion phase | Dispersion medium | Types of colloid | Examples |
| Solid | Solid | Solid sol | Some colored glasses and gemstones |
| Solid | Liquid | Sol | Paints, cell fluids |
| Solid | Gas | Aerosol | Smoke, dust |
| Liquid | Solid | Gel | Cheese, butter |
| Liquid | Liquid | Emulsion | Milk, hair cream |
| Liquid | Gas | Aerosol | Fog, cloud |
| Gas | Solid | Solid sol | Foam rubber |
| Gas | Liquid | Foam | Froth, soap lather |
-Now, let us discuss the options that are given to us.
-Fog is an aerosol. Fogs are formed in the atmosphere where the gas and liquid mix as dispersion medium and dispersed phase respectively.
-Shaving cream is a foam.
-Milk is Emulsion.
-Mud is a Sol.
-Jelly is a gel.
-Gemstones are Solid sols.
So, the correct answer is “Option D”.
Note: A gas mixed with other gas forms a homogeneous mixture; hence this system is not a colloidal system. The hydrophilic sols are more stable than hydrophobic sols because the stability of hydrophobic sols is due to charge only and hydrophilic sols are stable due to charge and solvation.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




