
Identify D.
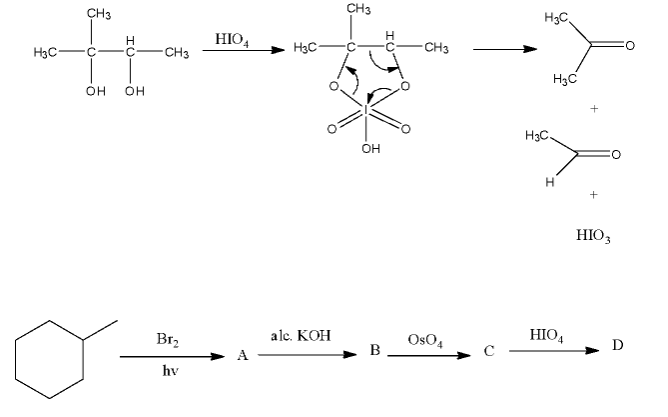
1,2-diols are oxidized to ketones or aldehydes by periodic acid $HI{{O}_{4}}$. Periodic acid reacts with diol to form a cyclic intermediate. The reaction takes place because iodine is in a highly positive oxidation state, so it readily accepts electrons. When the intermediate breaks down, the bond between the two carbons bonded to the –OH groups break.

Answer
579.3k+ views
Hint: Acyclic 1,2-doilos reacts with periodic acid (an oxidizing agent) and generally produces a ketone and an aldehyde compound as the products. Periodic acid is a good oxidizing agent and reacts with compounds having 1-2-diols in their structures.
Complete step by step solution:
- In the question it is given that 1,2-diols are oxidized to ketones or aldehydes by periodic acid.
- But as per the question we can say that the product C in the question will be a diol and it produces ketone or aldehydes as the product and we have to identify what C forms on reaction with periodic acid.
- The given chemical reaction is as follows.
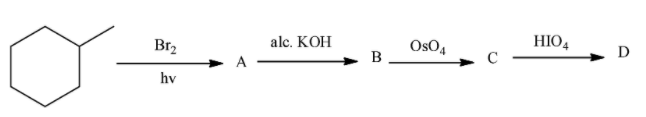
- First we have to identify A, B and C products in the above reaction.
- Reaction of methyl cyclohexane with bromine in sunlight is as follows.
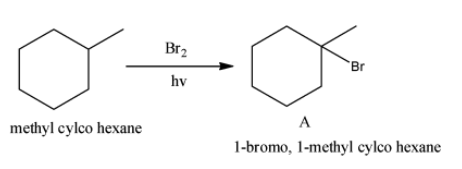
- Methyl cyclohexane reacts with bromine in presence of sunlight and produces 1-bromo, 1-methyl cyclohexane as the product.
- The product A reacts with alcoholic KOH and it is as follows.
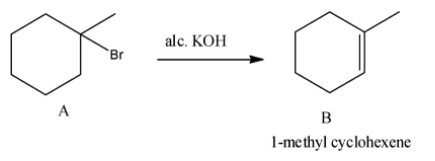
- 1-bromo, 1-methyl cyclohexane undergoes dehydrohalogenation in presence of alcoholic KOH and produces 1-methyl cyclohexene (B).
- 1-methyl cyclohexene (B) reacts with osmium tetroxide.
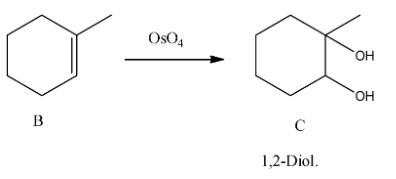
- Compound B reacts with osmium tetroxide and forms a 1,2-diol compound (C) as the product.
- Now compound C (1,2-diol) reacts with periodic acid.
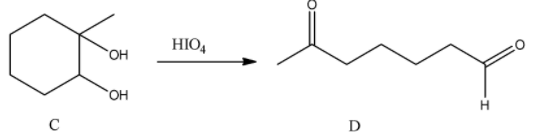
- Compound C reacts with periodic acid and produces a single product containing aldehyde functional group and ketone functional group in it.
Note: Aliphatic 1,2-doil compounds give more than one product (aldehydes and ketones) on reaction with periodic acid. But cyclic compounds give only one product having aldehyde and ketone functional groups in it.
Complete step by step solution:
- In the question it is given that 1,2-diols are oxidized to ketones or aldehydes by periodic acid.
- But as per the question we can say that the product C in the question will be a diol and it produces ketone or aldehydes as the product and we have to identify what C forms on reaction with periodic acid.
- The given chemical reaction is as follows.

- First we have to identify A, B and C products in the above reaction.
- Reaction of methyl cyclohexane with bromine in sunlight is as follows.

- Methyl cyclohexane reacts with bromine in presence of sunlight and produces 1-bromo, 1-methyl cyclohexane as the product.
- The product A reacts with alcoholic KOH and it is as follows.

- 1-bromo, 1-methyl cyclohexane undergoes dehydrohalogenation in presence of alcoholic KOH and produces 1-methyl cyclohexene (B).
- 1-methyl cyclohexene (B) reacts with osmium tetroxide.

- Compound B reacts with osmium tetroxide and forms a 1,2-diol compound (C) as the product.
- Now compound C (1,2-diol) reacts with periodic acid.

- Compound C reacts with periodic acid and produces a single product containing aldehyde functional group and ketone functional group in it.
Note: Aliphatic 1,2-doil compounds give more than one product (aldehydes and ketones) on reaction with periodic acid. But cyclic compounds give only one product having aldehyde and ketone functional groups in it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




