
Identify the true statements about the basic structure of amines:
A. Alkyl Amines are weaker bases than ammonia.
B. Arylamines are stronger bases than alkylamines.
C. Secondary aliphatic amines are stronger bases than primary aliphatic amines.
D. Tertiary aliphatic amines are weaker bases than arylamines.
Answer
606k+ views
Hint: Here we should know that the basicity of amines depends on the donation power of lone pairs present on the nitrogen atom, in their structure. You can draw the structures of representative elements of the options given and find the correct answer.
Complete step by step answer:
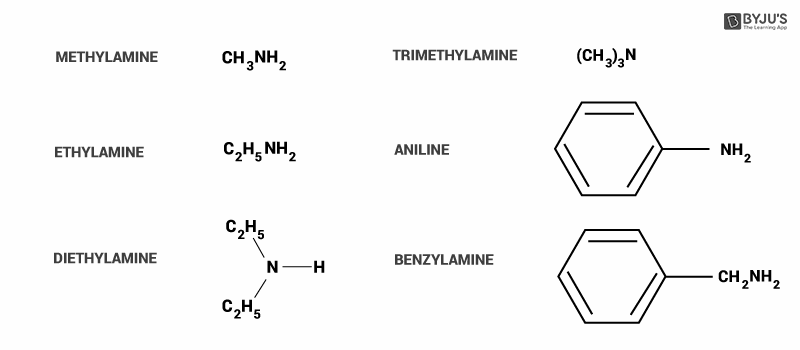
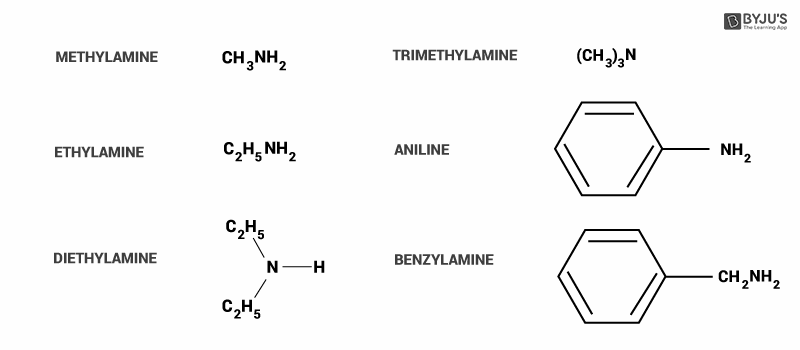
Here, we can see some common examples of amines.
In option A, alkylamines (methylamine) are stronger bases compared to ammonia because they have an alkyl group attached to them. It donates more electrons to the atom (+I effect), so that it can give more to act as a base. Hence this option is incorrect.
In option B, alkylamine (methylamine) is more basic than arylamine(aniline). In aniline, ring structure delocalized the electrons from individual atoms. The source of electrons is lone pair on the nitrogen atom.
The lone pair is also delocalized in aniline and resonance structures are formed as the lone pair on nitrogen is delocalized, it cannot donate an electron, therefore loses its basicity.
Methylamine does not have resonance and thus it is more basic as the Nitrogen can donate electrons. Hence, we can say that arylamines are weaker bases than alkylamines. So, this option is incorrect.
In option C, the statement “Secondary aliphatic amines are stronger bases than primary aliphatic amines” is correct because in secondary, nitrogen is attached with two alkyl groups that provide it more +I effect, hence, more charge on the atom. Therefore secondary aliphatic amine can donate more compared to the primary aliphatic amine.
In option D, as we discussed earlier, tertiary aliphatic amine would be having more +I effect, so more charge on the nitrogen atom, hence, it can donate more. That makes it more basic than arylamine. So, option D is incorrect.
Therefore, we can conclude that the correct answer to this question is option C.
Note: You can also arrange the compounds given in the increasing order of basicity -
Arylamine < tertiary aliphatic amine < primary aliphatic amine < secondary aliphatic amine
Here you can see some unusual behavior because there is some steric hindrance present in the structure of tertiary aliphatic amine, which makes the lone pair on nitrogen less available for donation. Hence its basicity is lesser compared to primary and secondary.
Complete step by step answer:


Here, we can see some common examples of amines.
In option A, alkylamines (methylamine) are stronger bases compared to ammonia because they have an alkyl group attached to them. It donates more electrons to the atom (+I effect), so that it can give more to act as a base. Hence this option is incorrect.
In option B, alkylamine (methylamine) is more basic than arylamine(aniline). In aniline, ring structure delocalized the electrons from individual atoms. The source of electrons is lone pair on the nitrogen atom.
The lone pair is also delocalized in aniline and resonance structures are formed as the lone pair on nitrogen is delocalized, it cannot donate an electron, therefore loses its basicity.
Methylamine does not have resonance and thus it is more basic as the Nitrogen can donate electrons. Hence, we can say that arylamines are weaker bases than alkylamines. So, this option is incorrect.
In option C, the statement “Secondary aliphatic amines are stronger bases than primary aliphatic amines” is correct because in secondary, nitrogen is attached with two alkyl groups that provide it more +I effect, hence, more charge on the atom. Therefore secondary aliphatic amine can donate more compared to the primary aliphatic amine.
In option D, as we discussed earlier, tertiary aliphatic amine would be having more +I effect, so more charge on the nitrogen atom, hence, it can donate more. That makes it more basic than arylamine. So, option D is incorrect.
Therefore, we can conclude that the correct answer to this question is option C.
Note: You can also arrange the compounds given in the increasing order of basicity -
Arylamine < tertiary aliphatic amine < primary aliphatic amine < secondary aliphatic amine
Here you can see some unusual behavior because there is some steric hindrance present in the structure of tertiary aliphatic amine, which makes the lone pair on nitrogen less available for donation. Hence its basicity is lesser compared to primary and secondary.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




