
If aniline is treated with conc. \[{H_2}S{O_4}\] and heated at ${200^\circ }C$, what would be the product?
A) Anilinium sulphate
B) Benzenesulfonic acid
C) m-aminobenzenesulfonic acid
D) sulphanilic acid
Answer
565.5k+ views
Hint:As we know that aromatic amines undergo typical electrophilic substitution reactions of aromatic ring and the amine group present on the ring is electron releasing and strongly ring activating and thereby increasing the electron density on ortho and para positions.
Complete step by step answer:
We are very well aware with the fact that aromatic amines undergo typical electrophilic substitution reaction of the benzene ring where the amine group activates the benzene ring by delocalisation of the lone pair of electrons present on the nitrogen atom towards the ring resulting in increase of electron density at ortho and para positions.
We also know that aniline undergoes sulfonation reaction where it reacts with concentrated sulphuric acid resulting in the formation of anilinium hydrogen sulphate as an intermediate which on further heating with sulphuric acid at a temperature of about $453 - 473K$ or simply at ${200^\circ }C$, produces a para-aminobenzene sulfonic acid which is commonly called as sulphanilic acid as the major product where $ - S{O_3}H$ group gets attached at the para position of the benzene ring. We can show this equation with the help of a chemical reaction as given below:
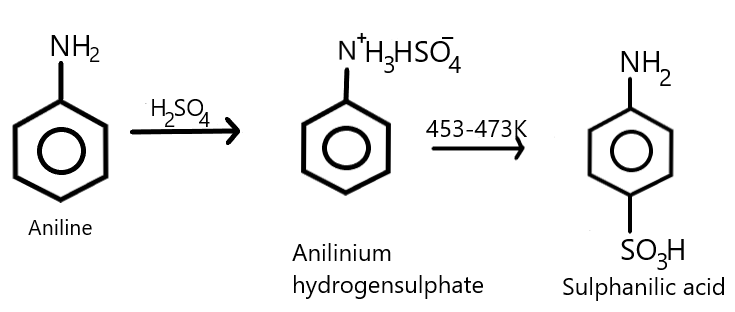
Therefore, from the above explanation we can say that the correct answer is (D).
Note:Always remember that $ - N{H_2}$ group present on the benzene ring is electron releasing and thus ortho (-o) and para (-p) directing towards electrophilic aromatic substitution reaction.
While direct nitration of aniline takes place in the presence of a strongly acidic medium, aniline is protonated to form anilinium ion which is meta- directing. Also on bromination of aniline, the major product is an ortho-para substituted bromoaniline because the benzene ring in this case is highly activated.
Complete step by step answer:
We are very well aware with the fact that aromatic amines undergo typical electrophilic substitution reaction of the benzene ring where the amine group activates the benzene ring by delocalisation of the lone pair of electrons present on the nitrogen atom towards the ring resulting in increase of electron density at ortho and para positions.
We also know that aniline undergoes sulfonation reaction where it reacts with concentrated sulphuric acid resulting in the formation of anilinium hydrogen sulphate as an intermediate which on further heating with sulphuric acid at a temperature of about $453 - 473K$ or simply at ${200^\circ }C$, produces a para-aminobenzene sulfonic acid which is commonly called as sulphanilic acid as the major product where $ - S{O_3}H$ group gets attached at the para position of the benzene ring. We can show this equation with the help of a chemical reaction as given below:

Therefore, from the above explanation we can say that the correct answer is (D).
Note:Always remember that $ - N{H_2}$ group present on the benzene ring is electron releasing and thus ortho (-o) and para (-p) directing towards electrophilic aromatic substitution reaction.
While direct nitration of aniline takes place in the presence of a strongly acidic medium, aniline is protonated to form anilinium ion which is meta- directing. Also on bromination of aniline, the major product is an ortho-para substituted bromoaniline because the benzene ring in this case is highly activated.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




