
In ${\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}$ every ${\text{Cr}}$ is linked to:
A) two O-atoms
B) three O-atoms
C) four O-atoms
D) five O-atoms
Answer
579.9k+ views
Hint: Dichromate ion has a chemical formula ${\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}$. Draw the structure of the dichromate ion and determine the number of an oxygen atom bonded with each ${\text{Cr}}$.
Complete step by step answer:
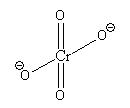
The prefix di stands for 2. So, the ion containing two chromate ions is known as dichromate ion. The structure of chromate ion (${\text{Cr}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$) is as follows:
In chromate ion, there is one ${\text{Cr}}$ atom bonded with 4 ${\text{O}}$ atoms.
The structure of the dichromate ion ${\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}$ is as follows:
In dichromate ion, there are 2 central ${\text{Cr}}$ atoms and 7 ${\text{O}}$ atoms.
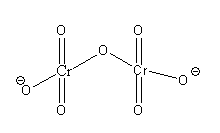
From the structure of dichromate ions, we can say that each central ${\text{Cr}}$ is bonded with 3 terminal oxygen atoms and 1 bride oxygen atom. Out of 7 oxygen atoms, 4 oxygen atoms are bonded with a double bond and 3 oxygen atoms are bonded with a single bond. Thus, each ${\text{Cr}}$ atom in dichromate ion is bonded with 4 oxygen atoms.
Hence, the correct option is (C) four O-atoms.
Additional Information: The bond angle of Cr-O-Cr is $126^\circ $. The bond length of bridge Cr-O is 179pm while the bond length of terminal Cr-O is 163 pm. The bond length of bridge Cr-O is greater than the bond length of the terminal Cr-O. This indicates all Cr-O bonds are not equal. The oxidation state of each Cr atom in dichromate ion is +6.
Note: Two tetrahedral chromate units share the oxygen atom and form dichromate ions. So, the number of oxygen atoms bonded with each ${\text{Cr}}$ atom in dichromate ion is the same as the number of oxygen atoms bonded with ${\text{Cr}}$ atom in chromate ion.
Complete step by step answer:
The prefix di stands for 2. So, the ion containing two chromate ions is known as dichromate ion. The structure of chromate ion (${\text{Cr}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$) is as follows:
In chromate ion, there is one ${\text{Cr}}$ atom bonded with 4 ${\text{O}}$ atoms.

The structure of the dichromate ion ${\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}$ is as follows:
In dichromate ion, there are 2 central ${\text{Cr}}$ atoms and 7 ${\text{O}}$ atoms.

From the structure of dichromate ions, we can say that each central ${\text{Cr}}$ is bonded with 3 terminal oxygen atoms and 1 bride oxygen atom. Out of 7 oxygen atoms, 4 oxygen atoms are bonded with a double bond and 3 oxygen atoms are bonded with a single bond. Thus, each ${\text{Cr}}$ atom in dichromate ion is bonded with 4 oxygen atoms.
Hence, the correct option is (C) four O-atoms.
Additional Information: The bond angle of Cr-O-Cr is $126^\circ $. The bond length of bridge Cr-O is 179pm while the bond length of terminal Cr-O is 163 pm. The bond length of bridge Cr-O is greater than the bond length of the terminal Cr-O. This indicates all Cr-O bonds are not equal. The oxidation state of each Cr atom in dichromate ion is +6.
Note: Two tetrahedral chromate units share the oxygen atom and form dichromate ions. So, the number of oxygen atoms bonded with each ${\text{Cr}}$ atom in dichromate ion is the same as the number of oxygen atoms bonded with ${\text{Cr}}$ atom in chromate ion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




