
In electrochemical cell, the oxidation and reduction occurs at:
A.Anode and cathode respectively
B.Cathode and anode respectively
C.Electrode
D.Anode
Answer
583.5k+ views
Hint: The reactions that produce or consume free electrons are known as electrochemistry. They are redox reactions where oxidation and reduction take place. An equal exchange of electrons takes place in an electrochemical reaction. Electrochemical reactions take place in an electrochemical cell. There are many applications. The most important of it is a battery.
Complete step by step answer:
Electrochemical cell involves two electrodes (cathode and anode) and an electrolyte.
The electrodes are different metals which are connected by a salt bridge.
As you already know an electrolyte can be a solution of water or other solvents in which the ions are mixed. An electrolyte is a compound that can dissociate into positively and negatively charged species, cations and anions respectively. For example, ${\text{KCl}} \to {{\text{K}}^{\text{ + }}}{\text{ + C}}{{\text{l}}^{\text{ - }}}$ . KCl solution is an electrolyte.
Now we need to know how an electrochemical cell works.
The 2 electrodes which are made up of metals are dipped in the separate electrolytic solution.
Let it be the Zn and Cu electrode.
As already mentioned before, electrodes are dipped in an electrolytic solution which can dissociate into cation and anion. Zn electrode in dipped in ${\text{ZnS}}{{\text{O}}_4}$ solution and Cu electrode dipped in ${\text{CuS}}{{\text{O}}_4}$ solution which can dissociate into ${\text{Z}}{{\text{n}}^{{\text{2 + }}}}$ and ${\text{C}}{{\text{u}}^{2 + }}$.
The basic thing we need to know is one metal should be an electron acceptor and others should donate electrons for the electrons to flow and to provide electric current.
Now, as we know according to the electrochemical series, Zn has a standard reduction value -0.76V whereas Cu has +0.34V. Thus Cu has a more reduction potential than zinc, which means that it can accept more electrons or pull electrons.
Since, Zn has less reduction potential, electrons from the Zn move to the ${\text{C}}{{\text{u}}^{2 + }}$ in the other solution. As electrons move from Zn to ${\text{C}}{{\text{u}}^{2 + }}$, there is loss of electrons at the Zn electrode forming ${\text{Z}}{{\text{n}}^{{\text{2 + }}}}$.
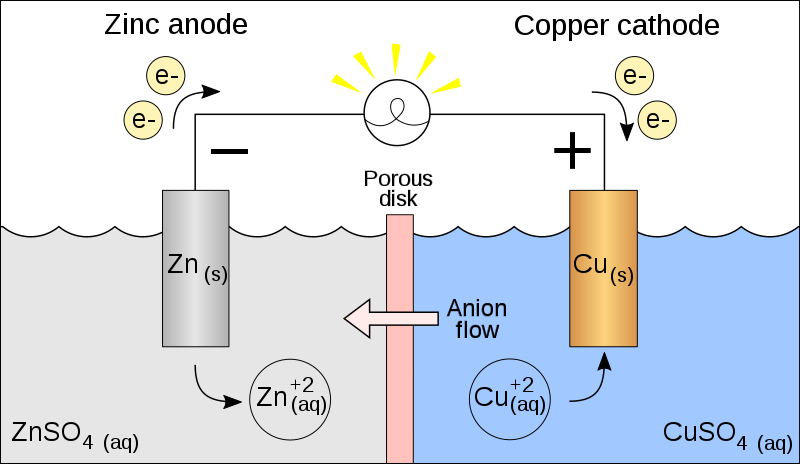
${\text{Zn}} \to {\text{Z}}{{\text{n}}^{{\text{2 + }}}}{\text{ + 2}}{{\text{e}}^{\text{ - }}}$ . So we can say that loss of electrons happens and Zn is said to be oxidized.
Similarly, ${\text{C}}{{\text{u}}^{2 + }}$ gains the ${\text{2}}{{\text{e}}^{\text{ - }}}$released by Zn and thus gets reduced ${\text{C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + 2}}{{\text{e}}^{\text{ - }}} \to {\text{Cu}}$
So here we can conclude that an electron is moving from Zn to Cu. This produces electricity.
As we know anode is a negatively charged electrode and a cathode is a positively charged electrode.
Electricity is produced when electrons flow from negative to positive. Thus, in this case, Zn is the anode and Cu is the cathode.
As already discussed above Zn is oxidized and Zinc is the anode as well.
So we can conclude that oxidation occurs at the anode and reduction occurs at the cathode.
Thus, the correct option is (A).
Note:In this question, we came across a term called an electrochemical series. It is known to be an activity series in which the elements are arranged based on their electrode potential values. This series represents the increasing order of the elements. The electrochemical series also tells about the decreasing reactivity of metals. Ongoing up the series, its ability to pull electrons increases.
Complete step by step answer:
Electrochemical cell involves two electrodes (cathode and anode) and an electrolyte.
The electrodes are different metals which are connected by a salt bridge.
As you already know an electrolyte can be a solution of water or other solvents in which the ions are mixed. An electrolyte is a compound that can dissociate into positively and negatively charged species, cations and anions respectively. For example, ${\text{KCl}} \to {{\text{K}}^{\text{ + }}}{\text{ + C}}{{\text{l}}^{\text{ - }}}$ . KCl solution is an electrolyte.
Now we need to know how an electrochemical cell works.
The 2 electrodes which are made up of metals are dipped in the separate electrolytic solution.
Let it be the Zn and Cu electrode.
As already mentioned before, electrodes are dipped in an electrolytic solution which can dissociate into cation and anion. Zn electrode in dipped in ${\text{ZnS}}{{\text{O}}_4}$ solution and Cu electrode dipped in ${\text{CuS}}{{\text{O}}_4}$ solution which can dissociate into ${\text{Z}}{{\text{n}}^{{\text{2 + }}}}$ and ${\text{C}}{{\text{u}}^{2 + }}$.
The basic thing we need to know is one metal should be an electron acceptor and others should donate electrons for the electrons to flow and to provide electric current.
Now, as we know according to the electrochemical series, Zn has a standard reduction value -0.76V whereas Cu has +0.34V. Thus Cu has a more reduction potential than zinc, which means that it can accept more electrons or pull electrons.
Since, Zn has less reduction potential, electrons from the Zn move to the ${\text{C}}{{\text{u}}^{2 + }}$ in the other solution. As electrons move from Zn to ${\text{C}}{{\text{u}}^{2 + }}$, there is loss of electrons at the Zn electrode forming ${\text{Z}}{{\text{n}}^{{\text{2 + }}}}$.

${\text{Zn}} \to {\text{Z}}{{\text{n}}^{{\text{2 + }}}}{\text{ + 2}}{{\text{e}}^{\text{ - }}}$ . So we can say that loss of electrons happens and Zn is said to be oxidized.
Similarly, ${\text{C}}{{\text{u}}^{2 + }}$ gains the ${\text{2}}{{\text{e}}^{\text{ - }}}$released by Zn and thus gets reduced ${\text{C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + 2}}{{\text{e}}^{\text{ - }}} \to {\text{Cu}}$
So here we can conclude that an electron is moving from Zn to Cu. This produces electricity.
As we know anode is a negatively charged electrode and a cathode is a positively charged electrode.
Electricity is produced when electrons flow from negative to positive. Thus, in this case, Zn is the anode and Cu is the cathode.
As already discussed above Zn is oxidized and Zinc is the anode as well.
So we can conclude that oxidation occurs at the anode and reduction occurs at the cathode.
Thus, the correct option is (A).
Note:In this question, we came across a term called an electrochemical series. It is known to be an activity series in which the elements are arranged based on their electrode potential values. This series represents the increasing order of the elements. The electrochemical series also tells about the decreasing reactivity of metals. Ongoing up the series, its ability to pull electrons increases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




