
In given reaction product P is :
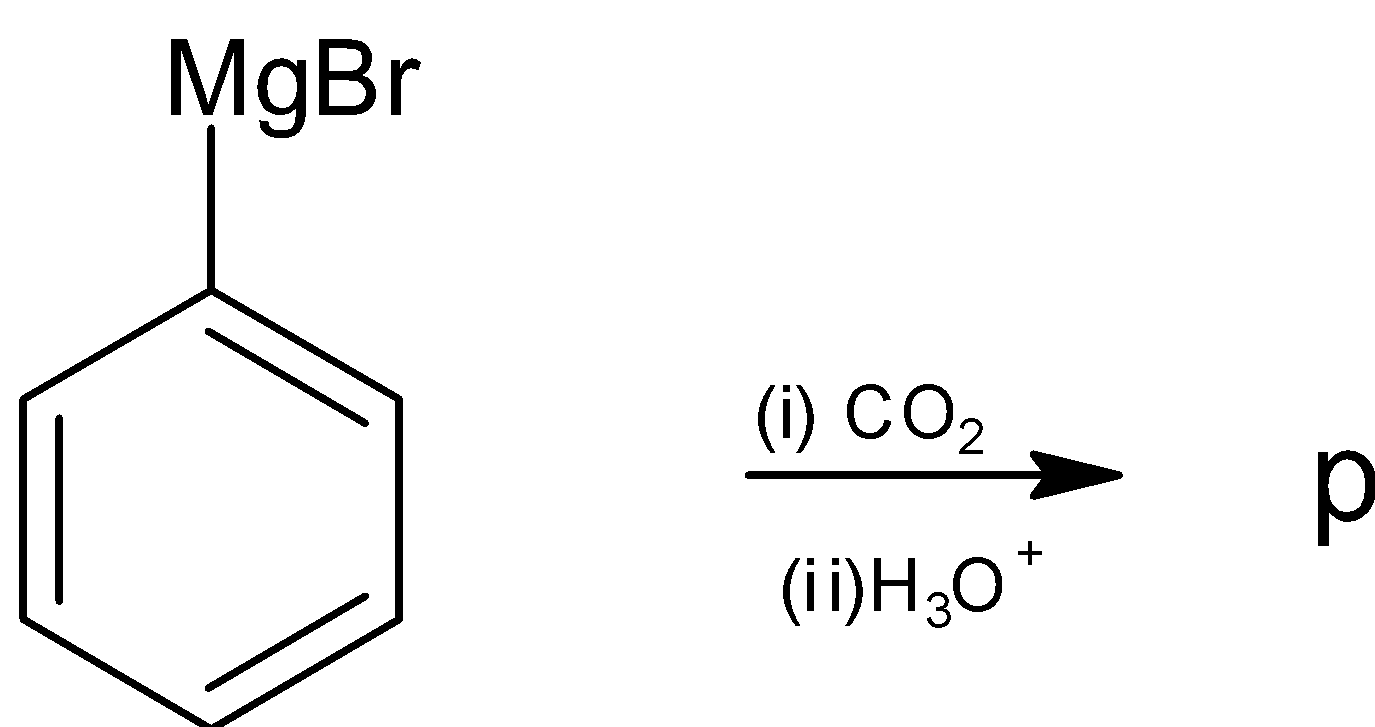
(a)
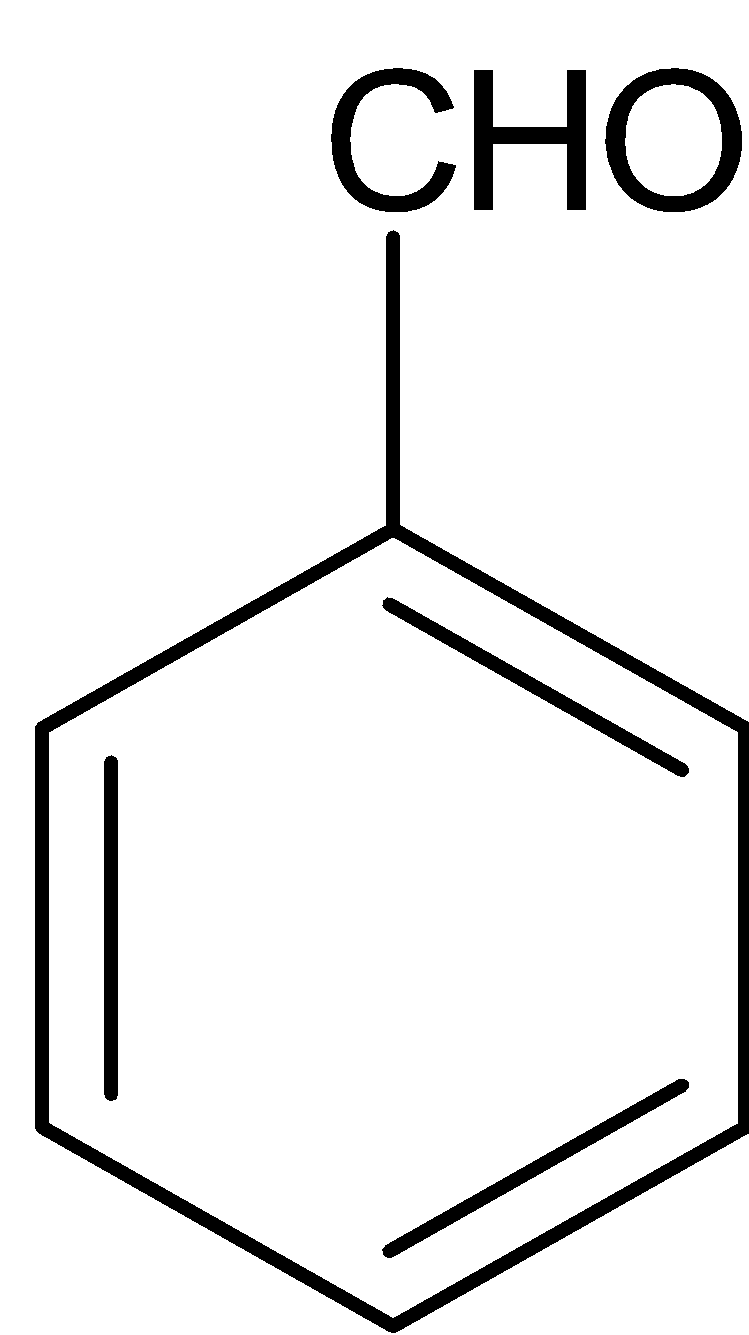
(b)
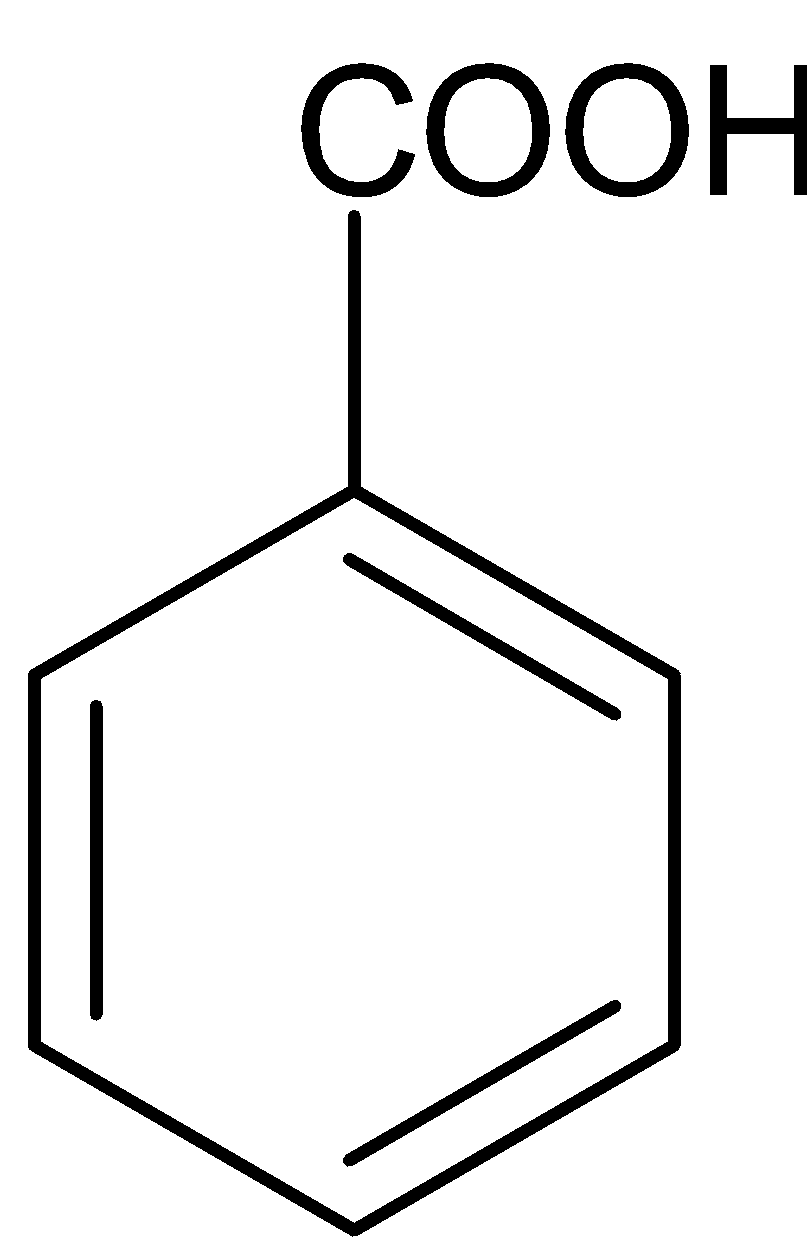
(c)
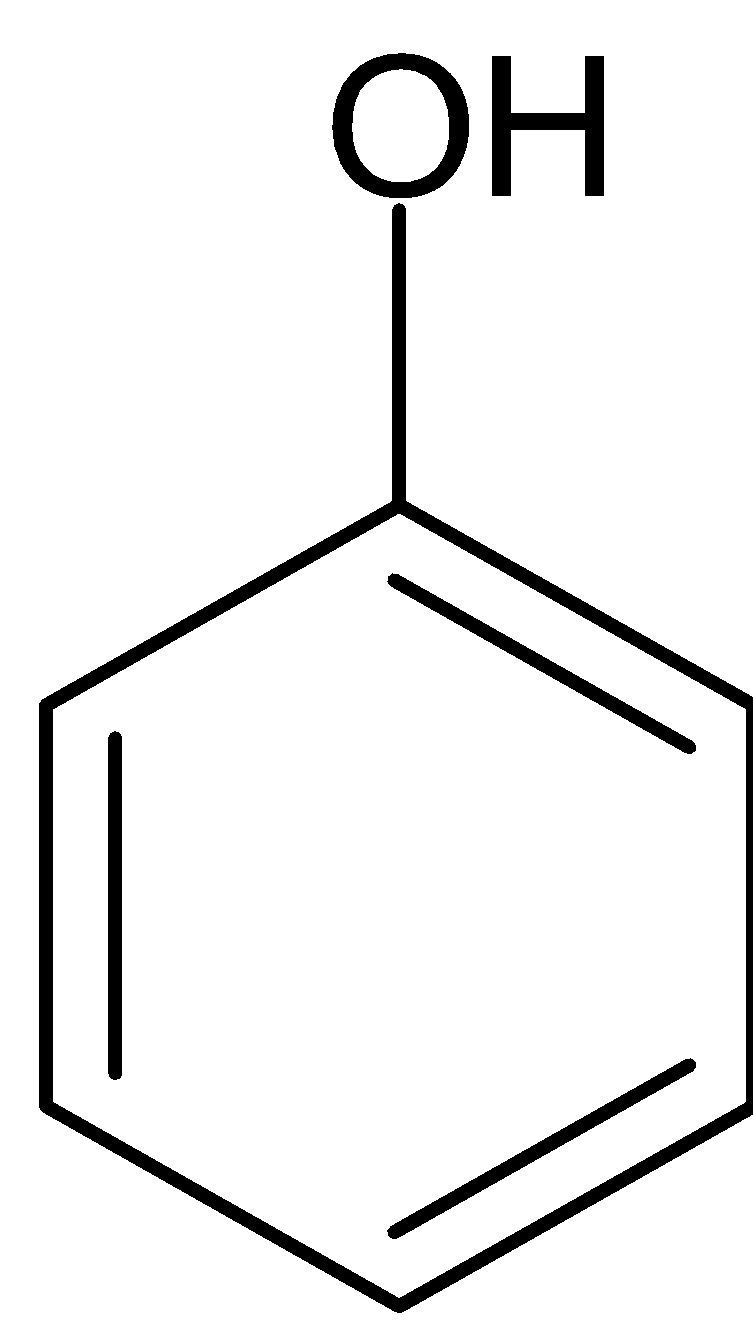




Answer
573.3k+ views
Hint: The chemical name of the reactant is phenylmagnesium bromide. Firstly we should understand the formation of phenylmagnesium bromide which will give us a better idea in answering this question.
Complete answer:
Firstly let's know the formation of phenylmagnesium bromide:
The bromobenzene is allowed to react with magnesium in presence ether to give phenylmagnesium bromide.
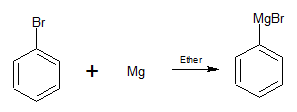
We can observe phenylmagnesium bromide is something similar to Grignard reagent.
-Now, let's see the reaction of phenylmagnesium bromide and carbon dioxide.
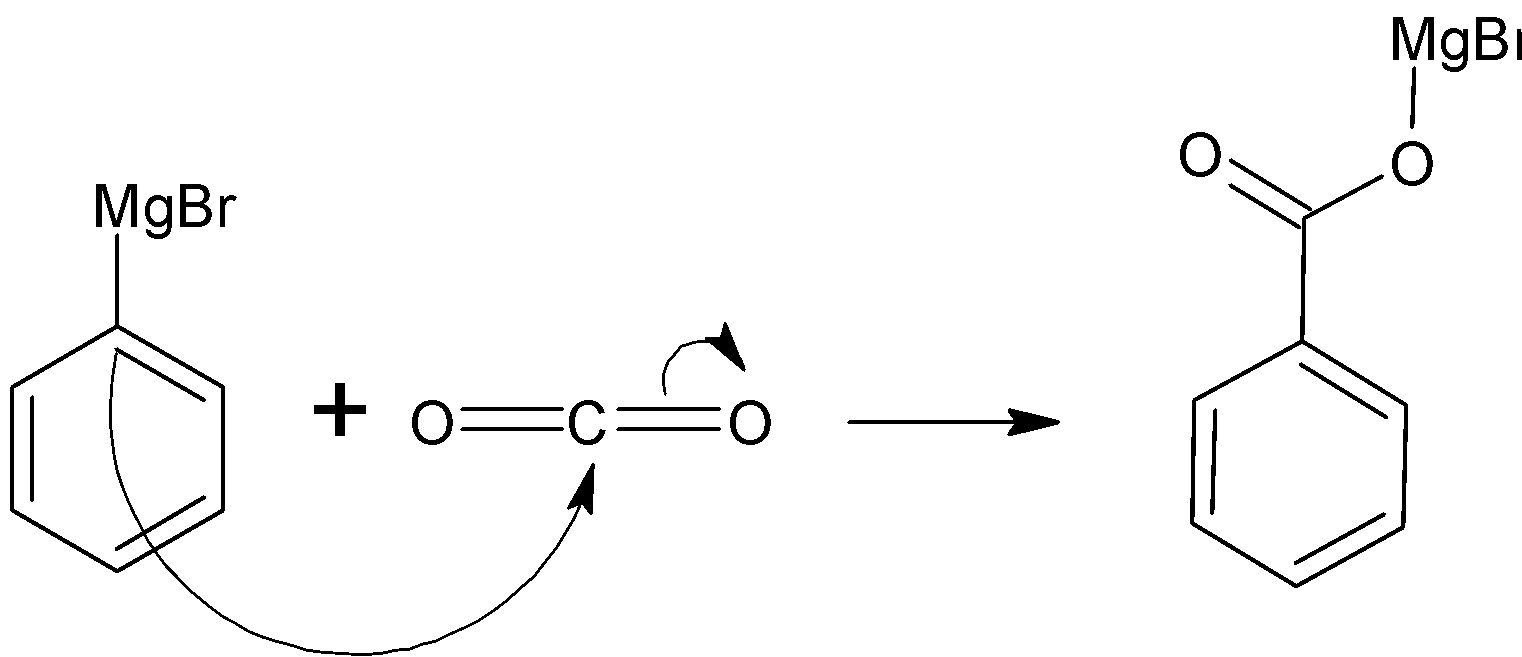
Since the carbon attached to phenylmagnesium bromide is partially negative. This carbon attacks the carbon of carbon dioxide. Just like nucleophilic addition . In this while the MgBr will be removed but since, the oxygen of carbon dioxide develops negative charge attracts the MgBr where the magnesium is positively charged resulting in formation of benzoate salt. Therefore, on reaction of phenylmagnesium bromide with carbon dioxide forms benzoate salt. -Followed by acid hydrolysis:
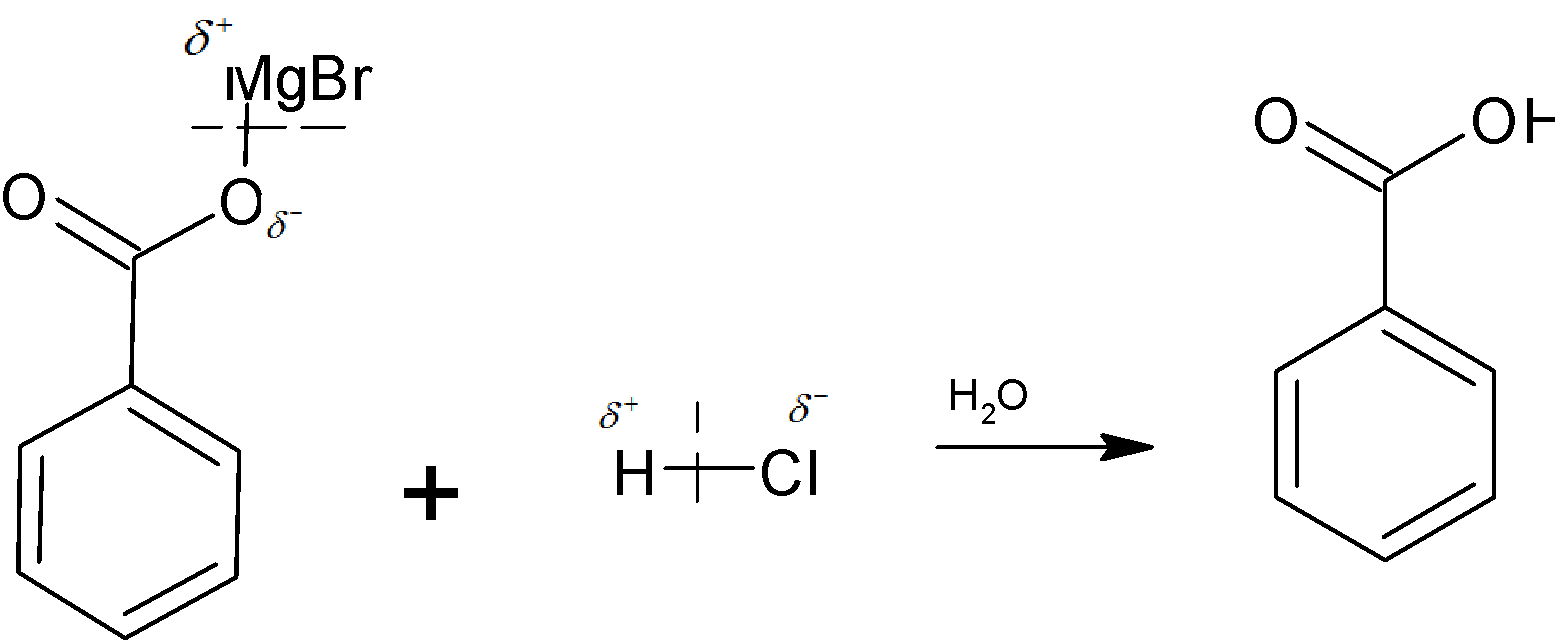
The hydrogen ion in the acid attacks the negatively charged oxygen to give an additional product. That is here hydrogen is the electrophile. The product formed is benzoic acid. This whole process is just like a nucleophilic addition reaction.
Thus, from the above discussion we can conclude that option B is the correct answer. The product P is benzoic acid.
Note: This reaction is similar to nucleophilic addition reaction. The carbon of phenylmagnesium bromide acts as the nucleophile here and carbon in carbon dioxide acts as the carbonyl carbon so, here phenylmagnesium bromide is the nucleophile.${{H}_{3}}{{O}^{+}}$ but the mixture of HCl and ${{H}_{2}}O.$
Complete answer:
Firstly let's know the formation of phenylmagnesium bromide:
The bromobenzene is allowed to react with magnesium in presence ether to give phenylmagnesium bromide.
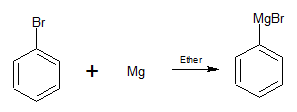
We can observe phenylmagnesium bromide is something similar to Grignard reagent.
-Now, let's see the reaction of phenylmagnesium bromide and carbon dioxide.

Since the carbon attached to phenylmagnesium bromide is partially negative. This carbon attacks the carbon of carbon dioxide. Just like nucleophilic addition . In this while the MgBr will be removed but since, the oxygen of carbon dioxide develops negative charge attracts the MgBr where the magnesium is positively charged resulting in formation of benzoate salt. Therefore, on reaction of phenylmagnesium bromide with carbon dioxide forms benzoate salt. -Followed by acid hydrolysis:

The hydrogen ion in the acid attacks the negatively charged oxygen to give an additional product. That is here hydrogen is the electrophile. The product formed is benzoic acid. This whole process is just like a nucleophilic addition reaction.
Thus, from the above discussion we can conclude that option B is the correct answer. The product P is benzoic acid.
Note: This reaction is similar to nucleophilic addition reaction. The carbon of phenylmagnesium bromide acts as the nucleophile here and carbon in carbon dioxide acts as the carbonyl carbon so, here phenylmagnesium bromide is the nucleophile.${{H}_{3}}{{O}^{+}}$ but the mixture of HCl and ${{H}_{2}}O.$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




