
In halogenation of aromatic hydrocarbon, a halogen carrier is used which is generally a Lewis acid. The main function of this species is to generate the species:
[A] X
[B] ${{X}^{-}}$
[C] ${{X}^{+}}$
[D] $X\centerdot $
Answer
578.4k+ views
Hint: To answer this, think about chlorination or bromination of benzene or other benzene derived species. Remember that the reaction proceeds via aromatic electrophilic substitution mechanism. Also, remember that Lewis acids are electron deficient species.
Complete answer:
To answer this, firstly let us discuss the reaction mechanism of halogenation of aromatic hydrocarbons.
Halogenation of aromatic hydrocarbons proceeds through an electrophilic substitution pathway and since it includes aromatic hydrocarbons, the reaction is known as electrophilic aromatic substitution.
For reactive hydrocarbons like phenol, no catalyst is needed but for less reactive benzene derivatives we use a Lewis acid catalyst like $AlC{{l}_{3}},FeC{{l}_{3}},AgB{{r}_{3}}\text{ and ZnC}{{\text{l}}_{3}}$. The use of these catalysts is the formation of highly electrophilic complexes which are attacked by the benzene ring and leads to halogenation.
We know that electrophiles are electron deficient species i.e. they can accept a pair of electrons readily.
Let us see the reaction mechanism to understand it better.
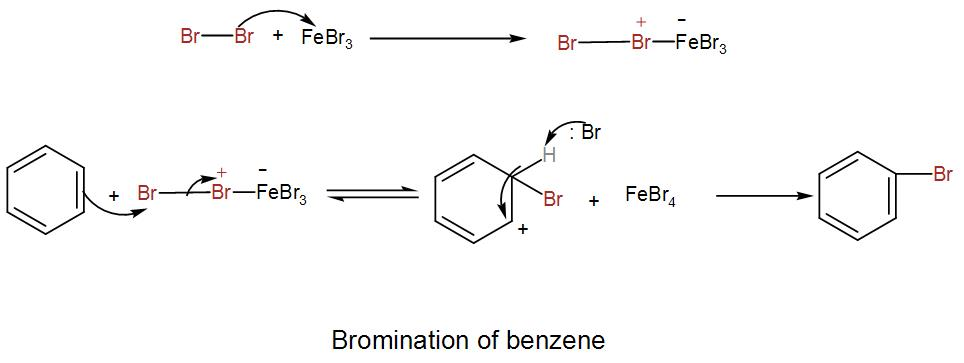
We can understand from the above discussion that the Lewis acid is responsible for the formation of the electrophilic species.
Therefore, the correct answer is option [C] ${{X}^{+}}$
Note:
The Lewis theory states that “Lewis acid is a species which can accept a pair of electrons to form a coordinate covalent bond. Lewis base is a species which can donate a pair of electrons to form a coordinate covalent bond.”
We can describe the characteristics of species which are Lewis acid as-
(i) Molecules containing an atom with incomplete valence shell like $B{{F}_{3}}$.
(ii) Metal atoms or cations having empty or partially filled orbitals like nickel or silver etc.
(iii) The molecule or ion capable of accepting electron pairs by rearrangement of its valence electrons.
(iv) The central atom or some molecule may expand its octet to accommodate electron pairs for example $Si{{F}_{4}}$.
Complete answer:
To answer this, firstly let us discuss the reaction mechanism of halogenation of aromatic hydrocarbons.
Halogenation of aromatic hydrocarbons proceeds through an electrophilic substitution pathway and since it includes aromatic hydrocarbons, the reaction is known as electrophilic aromatic substitution.
For reactive hydrocarbons like phenol, no catalyst is needed but for less reactive benzene derivatives we use a Lewis acid catalyst like $AlC{{l}_{3}},FeC{{l}_{3}},AgB{{r}_{3}}\text{ and ZnC}{{\text{l}}_{3}}$. The use of these catalysts is the formation of highly electrophilic complexes which are attacked by the benzene ring and leads to halogenation.
We know that electrophiles are electron deficient species i.e. they can accept a pair of electrons readily.
Let us see the reaction mechanism to understand it better.

We can understand from the above discussion that the Lewis acid is responsible for the formation of the electrophilic species.
Therefore, the correct answer is option [C] ${{X}^{+}}$
Note:
The Lewis theory states that “Lewis acid is a species which can accept a pair of electrons to form a coordinate covalent bond. Lewis base is a species which can donate a pair of electrons to form a coordinate covalent bond.”
We can describe the characteristics of species which are Lewis acid as-
(i) Molecules containing an atom with incomplete valence shell like $B{{F}_{3}}$.
(ii) Metal atoms or cations having empty or partially filled orbitals like nickel or silver etc.
(iii) The molecule or ion capable of accepting electron pairs by rearrangement of its valence electrons.
(iv) The central atom or some molecule may expand its octet to accommodate electron pairs for example $Si{{F}_{4}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




