
In pyrophosphoric acid, ${{H}_{4}}{{P}_{2}}{{O}_{7}}$, number of $\sigma -$ and $d\pi -p\pi $ bonds respectively are:
A. 8 and 2
B. 6 and 2
C. 12 and 0
D. 12 and 2
Answer
594k+ views
Hint: Draw the structure of the molecule,${{H}_{4}}{{P}_{2}}{{O}_{7}}$. Make sure the valencies of all the atoms are satisfied. Remember that $P$ makes 5 bonds with the surrounding atoms, $O$ makes 2 bonds, and $H$ makes 1 bond.
Complete answer:
Let us consider the structure of pyrophosphoric acid:
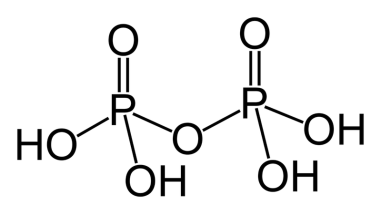
$\sigma -$bonds
-Each phosphorus atom is bonded to 2 $-OH$ which makes 4 $P-OH$sigma bonds.
- Each phosphorus atom is bonded to 2 $O$ atoms which makes 4 $P-O$ sigma bonds.
- Since there are 4 $-OH$ present, 4 $O-H$ sigma bonds are present.
- By adding the number of sigma bonds in these three points we see that the total number of sigma bonds is 12.
Now onto the $d\pi -p\pi $bonds
- Each phosphorus atom shares a double bond with one $O$ atom, which makes 2 $P=O$ bonds.
- There are no more double bonds to take into account.
- The number of $d\pi -p\pi $ present are 2.
Therefore, the answer to this question is D. 12 and 2
Additional Information:
The name pyrophosphoric acid was coined due to the way in which the chemical is formed. When phosphoric acid is heated to red heat, it was found that pyrophosphoric acid is formed. This is also an easier way to memorize the molecular structure of this compound.
Note: Please do not confuse the answer with ‘A. 8 and 2’. It is very easy to overlook the bonds that are involved in the $O-H$ system since these bonds are not shown in most images. Make sure that you have considered all the atoms and what bonds they form before writing down the answer.
Complete answer:
Let us consider the structure of pyrophosphoric acid:

$\sigma -$bonds
-Each phosphorus atom is bonded to 2 $-OH$ which makes 4 $P-OH$sigma bonds.
- Each phosphorus atom is bonded to 2 $O$ atoms which makes 4 $P-O$ sigma bonds.
- Since there are 4 $-OH$ present, 4 $O-H$ sigma bonds are present.
- By adding the number of sigma bonds in these three points we see that the total number of sigma bonds is 12.
Now onto the $d\pi -p\pi $bonds
- Each phosphorus atom shares a double bond with one $O$ atom, which makes 2 $P=O$ bonds.
- There are no more double bonds to take into account.
- The number of $d\pi -p\pi $ present are 2.
Therefore, the answer to this question is D. 12 and 2
Additional Information:
The name pyrophosphoric acid was coined due to the way in which the chemical is formed. When phosphoric acid is heated to red heat, it was found that pyrophosphoric acid is formed. This is also an easier way to memorize the molecular structure of this compound.
Note: Please do not confuse the answer with ‘A. 8 and 2’. It is very easy to overlook the bonds that are involved in the $O-H$ system since these bonds are not shown in most images. Make sure that you have considered all the atoms and what bonds they form before writing down the answer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




