
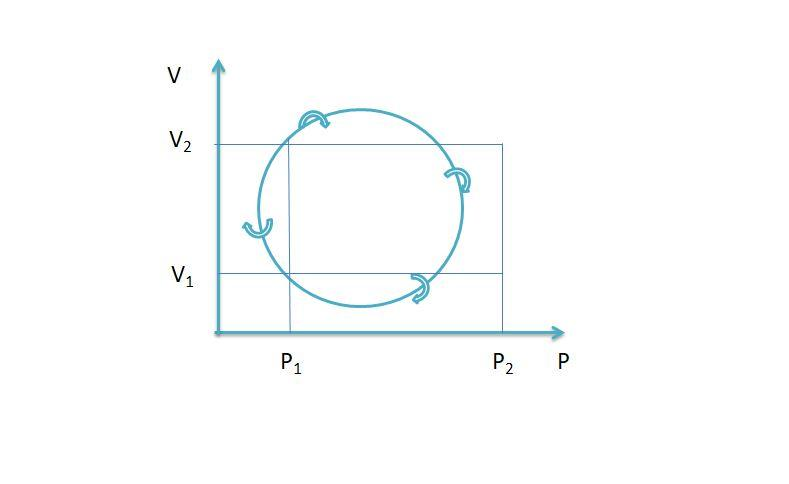
In the cyclic process shown in P-V diagram, the magnitude of the work done is
A .$\pi {\dfrac{{({P_2} - {P_1})}}{2}^2}$
B .$\dfrac{{\pi {{({V_2} - {V_1})}^2}}}{2}$
C .$\dfrac{\pi }{4}\left( {{P_2} - {P_1}} \right)\left( {{V_2} - {V_1}} \right)$
D .$\pi \left( {{P_2}{P_1} - {V_2}{V_1}} \right)$

Answer
579.6k+ views
Hint: The internal energy of the system will not change since it is a cyclic process the work will be done in this process.
Acyclic system is the one in which the initial and the final state is the same the i.e: the system starts and ends at the same state in a thermodynamic process
Complete step by step answer:
In order to calculate the net work done by the system we need to analyse each and every step in detail:
Step 1: Since the internal energy does not change therefore the system does the work W1 equivalent to the heat Q1 added to the system during the isothermal expansion.
Step 2: The work done W2=0 during the Isochoric process.
Step 3: The work done W3 is smaller in magnitude and is negative as well since this work is done by the system during the isothermal compression process as shown by the area under the PV curve in step 1 which is higher in magnitude as compared to that in the step 3. Here in this step Q3= W3 as the internal energy of the system does not change.
Step 4: This is the reverse of the step 2 involved in the process and is known as the Isochoric process. The heat Q4= -Q2 is the heat added to the system and the work done W4 done is =0.
Thus $\dfrac{\pi }{4}\left( {{P_2} - {P_1}} \right)\left( {{V_2} - {V_1}} \right)$is the network done in the cyclic process.
So, the correct answer is Option C.
Note:
The area enclosed by the P-V diagram is the net work done in the cyclic process. The work is done on the system if the system operates anticlockwise and the work is done by the system if the system operates clockwise.
Acyclic system is the one in which the initial and the final state is the same the i.e: the system starts and ends at the same state in a thermodynamic process
Complete step by step answer:
In order to calculate the net work done by the system we need to analyse each and every step in detail:
Step 1: Since the internal energy does not change therefore the system does the work W1 equivalent to the heat Q1 added to the system during the isothermal expansion.
Step 2: The work done W2=0 during the Isochoric process.
Step 3: The work done W3 is smaller in magnitude and is negative as well since this work is done by the system during the isothermal compression process as shown by the area under the PV curve in step 1 which is higher in magnitude as compared to that in the step 3. Here in this step Q3= W3 as the internal energy of the system does not change.
Step 4: This is the reverse of the step 2 involved in the process and is known as the Isochoric process. The heat Q4= -Q2 is the heat added to the system and the work done W4 done is =0.
Thus $\dfrac{\pi }{4}\left( {{P_2} - {P_1}} \right)\left( {{V_2} - {V_1}} \right)$is the network done in the cyclic process.
So, the correct answer is Option C.
Note:
The area enclosed by the P-V diagram is the net work done in the cyclic process. The work is done on the system if the system operates anticlockwise and the work is done by the system if the system operates clockwise.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




