
In the following reaction, ${{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\xrightarrow[(ii){{H}_{3}}{{O}^{+}}]{(i)Mg,\,ether}X$, the product ‘X’ is:
(A) ${{C}_{6}}{{H}_{5}}C{{H}_{2}}OC{{H}_{2}}{{C}_{6}}{{H}_{5}}$
(B) ${{C}_{6}}{{H}_{5}}C{{H}_{2}}OH$
(C) ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$
(D) ${{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{H}_{2}}{{C}_{6}}{{H}_{5}}$
Answer
587.7k+ views
Hint: A halogen derivative of alkane reacts with magnesium metal to form a very famous reagent. That reagent is an organometallic compound having a central magnesium atom directly bonded to an alkyl group and a halogen atom.
Complete step by step solution:
-Grignard reagent is an organometallic compound having a magnesium atom directly bonded to an alkyl group and a halogen atom.
-An organometallic compound is a compound in which a metal atom is directly bonded to a carbon atom. Some examples of organometallic compounds are methyl lithium, methyl magnesium bromide, trimethylaluminum, etc.
-When an alkyl halide reacts with magnesium metal in the presence of dry conditions which is provided using dry ether, alkyl magnesium halide is formed. This alkyl magnesium halide is known as Grignard’s reagent.
-In this case, benzyl bromide first reacts with magnesium metal in the presence of dry ether to form Benzyl magnesium bromide.
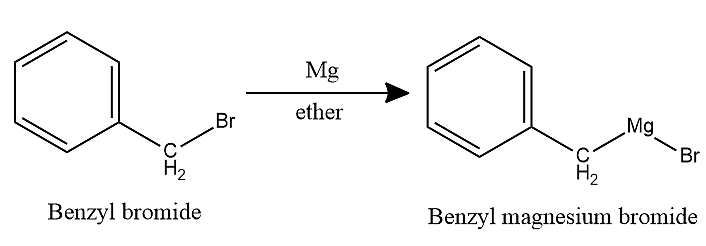
-Then, Benzyl magnesium bromide undergoes hydrolysis to give toluene.
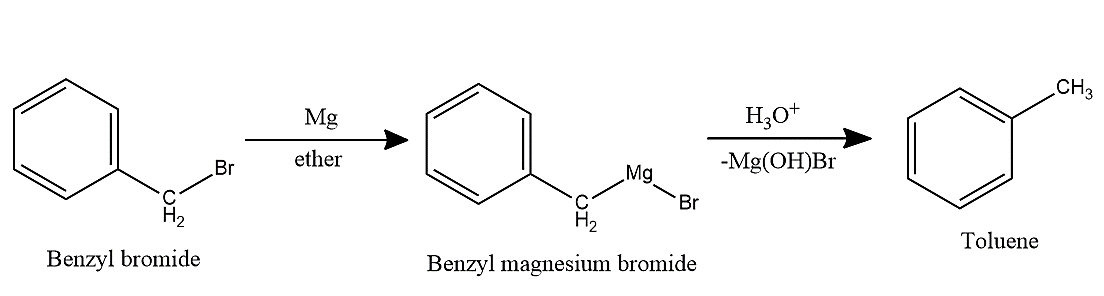
-We can also represent this reaction as follows,
${{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\xrightarrow[(ii){{H}_{3}}{{O}^{+}}]{(i)Mg,\,ether}{{C}_{6}}{{H}_{5}}C{{H}_{3}}$
Therefore, in the reaction, ${{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\xrightarrow[(ii){{H}_{3}}{{O}^{+}}]{(i)Mg,\,ether}X$, the product ‘X’ is Toluene.
So, the correct answer is option (C) ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$
Note: Carefully read these types of questions. The order in which the reactants present on the reaction arrow react with the starting compound is very important. When the numbers are reversed then the reaction might not take place or products obtained will be different.
Complete step by step solution:
-Grignard reagent is an organometallic compound having a magnesium atom directly bonded to an alkyl group and a halogen atom.
-An organometallic compound is a compound in which a metal atom is directly bonded to a carbon atom. Some examples of organometallic compounds are methyl lithium, methyl magnesium bromide, trimethylaluminum, etc.
-When an alkyl halide reacts with magnesium metal in the presence of dry conditions which is provided using dry ether, alkyl magnesium halide is formed. This alkyl magnesium halide is known as Grignard’s reagent.
-In this case, benzyl bromide first reacts with magnesium metal in the presence of dry ether to form Benzyl magnesium bromide.

-Then, Benzyl magnesium bromide undergoes hydrolysis to give toluene.
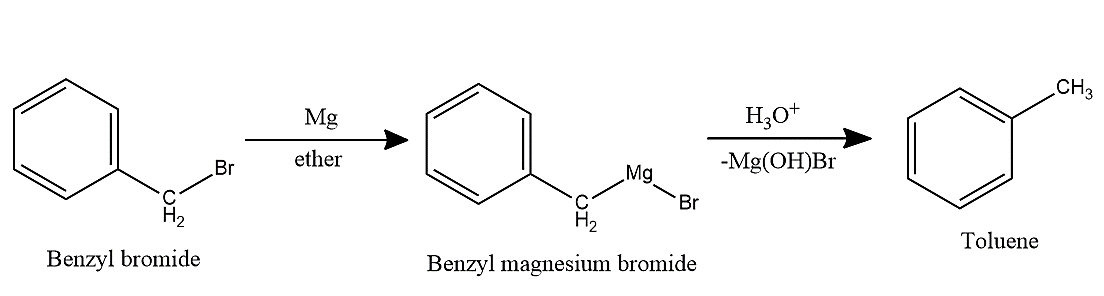
-We can also represent this reaction as follows,
${{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\xrightarrow[(ii){{H}_{3}}{{O}^{+}}]{(i)Mg,\,ether}{{C}_{6}}{{H}_{5}}C{{H}_{3}}$
Therefore, in the reaction, ${{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\xrightarrow[(ii){{H}_{3}}{{O}^{+}}]{(i)Mg,\,ether}X$, the product ‘X’ is Toluene.
So, the correct answer is option (C) ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$
Note: Carefully read these types of questions. The order in which the reactants present on the reaction arrow react with the starting compound is very important. When the numbers are reversed then the reaction might not take place or products obtained will be different.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




