
In water, the angle between the atoms of the hydrogen and one atom of oxygen is
(a) ${ 180 }^{ ° }$
(b)${ 104.5 }^{ ° }$
(c)${ 106.5 }^{ ° }$
(d)${ 154.8 }^{ ° }$
Answer
579.9k+ views
Hint: In water, two hydrogen atoms and one oxygen atom attached by two sigma bonds and with 2 lone pairs of electrons around the oxygen atom, so the bond angle between the hydrogen atoms.
Complete answer:
The oxygen is more electronegative than hydrogen, the hydrogen atoms end up with a partial positive charge while the oxygen atom with a partial negative charge and a net dipole moment on the molecule produces due to this separation of charge. The structure of the water molecule leads to hydrogen bonding, which is a stabilized structure in which a hydrogen atom is in a line between the oxygen atom on its molecule and the oxygen on another molecule. A hydrogen-bonded structure between two water molecules can be seen. With their extra attractive energy, these hydrogen bonds are the cause of many of the unusual properties of water that includes large heat of vaporization and its expansion upon freezing. The angle between hydrogen atoms in the water molecule is approximate ${ 105 }^{ ° }$ which is the lowest energy configuration for the system. In the water molecule, the resulting angle occurs due to the oxygen atom having two sets of lone pair electrons with interacting repulsive fields. They consequently push the two hydrogen atoms closer together since the lone pairs are mutually repulsed and tend to move away from each other. The three atoms 2H and O make an angle which is approximately 104.5 degrees and the center of each hydrogen atom is approximately 0.0957 nm from the center of the oxygen atom.
Additional Information: 1)The covalent bonds between hydrogen and oxygen atoms in water are polar covalent bonds. The electrons give it a small negative charge as they share more time near the oxygen nucleus, than they spend near the hydrogen nuclei, giving these molecules a small positive charge.
2) In a water molecule, two hydrogen atoms are covalently bonded to the oxygen atom which means water molecules tend to form weak bonds with other water molecules. It is because the oxygen end of the molecule is negative while the hydrogen ends are positive.
3) Because of its polar covalent bonds water is classified as a polar molecule and it has a bent shape.
So, the correct answer is, ‘${ 104.5 }^{ ° }$’.
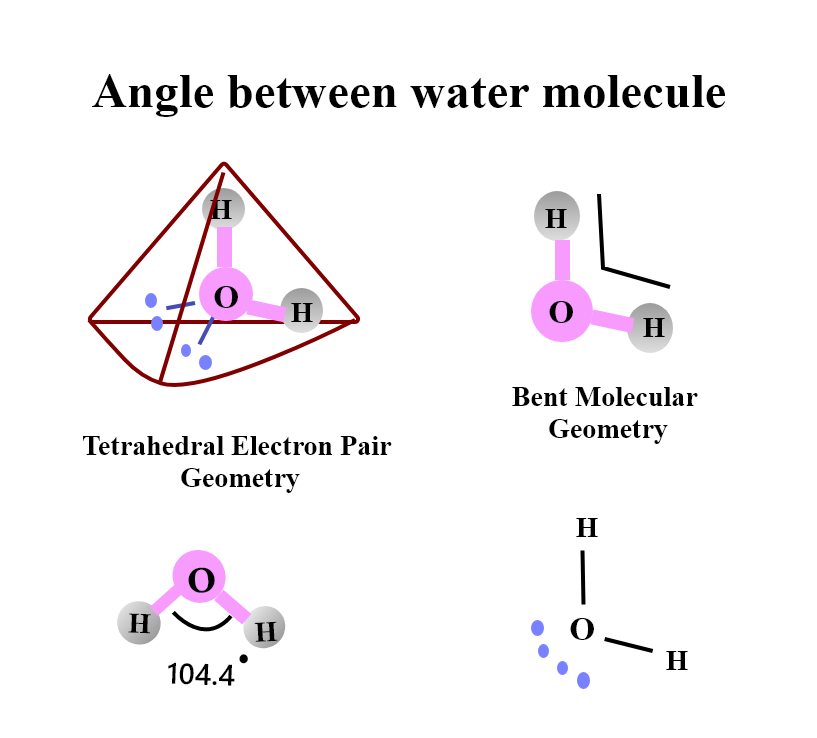
Note: The bonds between ${ H }_{ 2 }{ O }$ by the Lewis structure and valence bond theory describes as two sigma bonds between the central oxygen atom and the two peripheral hydrogen atoms with oxygen having two lone pairs of electrons suggests that ${ H }_{ 2 }{ O }$ is sp3 hybridized. Here to form four new hybridized orbitals the 2s atomic orbital and the three 2p orbitals of oxygen are hybridized which then participate in bonding by overlapping with the hydrogen 1s orbitals. The tetrahedral is predictable shape and bond angle as they have ${ sp }^{ 3 }$ hybridization and ${ 109.5 }^{ ° }$ which is an open agreement with the true bond angle of ${ 104.5 }^{ ° }$.
Complete answer:
The oxygen is more electronegative than hydrogen, the hydrogen atoms end up with a partial positive charge while the oxygen atom with a partial negative charge and a net dipole moment on the molecule produces due to this separation of charge. The structure of the water molecule leads to hydrogen bonding, which is a stabilized structure in which a hydrogen atom is in a line between the oxygen atom on its molecule and the oxygen on another molecule. A hydrogen-bonded structure between two water molecules can be seen. With their extra attractive energy, these hydrogen bonds are the cause of many of the unusual properties of water that includes large heat of vaporization and its expansion upon freezing. The angle between hydrogen atoms in the water molecule is approximate ${ 105 }^{ ° }$ which is the lowest energy configuration for the system. In the water molecule, the resulting angle occurs due to the oxygen atom having two sets of lone pair electrons with interacting repulsive fields. They consequently push the two hydrogen atoms closer together since the lone pairs are mutually repulsed and tend to move away from each other. The three atoms 2H and O make an angle which is approximately 104.5 degrees and the center of each hydrogen atom is approximately 0.0957 nm from the center of the oxygen atom.
Additional Information: 1)The covalent bonds between hydrogen and oxygen atoms in water are polar covalent bonds. The electrons give it a small negative charge as they share more time near the oxygen nucleus, than they spend near the hydrogen nuclei, giving these molecules a small positive charge.
2) In a water molecule, two hydrogen atoms are covalently bonded to the oxygen atom which means water molecules tend to form weak bonds with other water molecules. It is because the oxygen end of the molecule is negative while the hydrogen ends are positive.
3) Because of its polar covalent bonds water is classified as a polar molecule and it has a bent shape.
So, the correct answer is, ‘${ 104.5 }^{ ° }$’.

Note: The bonds between ${ H }_{ 2 }{ O }$ by the Lewis structure and valence bond theory describes as two sigma bonds between the central oxygen atom and the two peripheral hydrogen atoms with oxygen having two lone pairs of electrons suggests that ${ H }_{ 2 }{ O }$ is sp3 hybridized. Here to form four new hybridized orbitals the 2s atomic orbital and the three 2p orbitals of oxygen are hybridized which then participate in bonding by overlapping with the hydrogen 1s orbitals. The tetrahedral is predictable shape and bond angle as they have ${ sp }^{ 3 }$ hybridization and ${ 109.5 }^{ ° }$ which is an open agreement with the true bond angle of ${ 104.5 }^{ ° }$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




