
In which of the following pairs both angle strain and torsional strain is present?
A: Cyclopropane , Cyclobutane
B: Cyclopropane, Cyclohexane
C: Cyclobutane , Cyclohexane
D: Cyclohexane , Cyclopentane
Answer
583.2k+ views
Hint:Torsional strain is a strain which occurs due to the increase in the potential energy of the molecule . It occurs due to the repulsion between electrons in bonds that do not share an atom. Angle strain is the increase in potential energy due to the deviated bond angles.
Complete step by step answer:In the given question we have to find which pair of the cycloalkanes have both the torsional and angle strain. Angle strain occurs when there is a deviation of the bond angles from the ideal bond angles. It basically increases the potential energy of the molecule. The smaller cycloalkanes have more torsional strain as compared to the larger cyclohexane. In smaller cycloalkane the hydrogens eclipse each other. In cycloalkane the carbon atom is of $s{p_3}$ hybridisation. The angle of a $s{p_3}$ hybridised atom is $109.5^\circ $ . But in cyclopropane the carbon atoms are maintained in $60^\circ $ . This means that it has an angle strain of $49.5^\circ $ , which is very high. The carbon atoms of the cyclobutene are maintained in $90^\circ $ . The angle strain in cyclobutane is around $19.5^\circ $ . On the other hand, the angle strain of cyclohexane and cyclopentane is negligible. So according to the above explanation it is very much clear to us that cyclopropane and cyclobutane have both torsional and angle strain .
So the correct answer of the above question is :
A: Cyclopropane , Cyclobutane
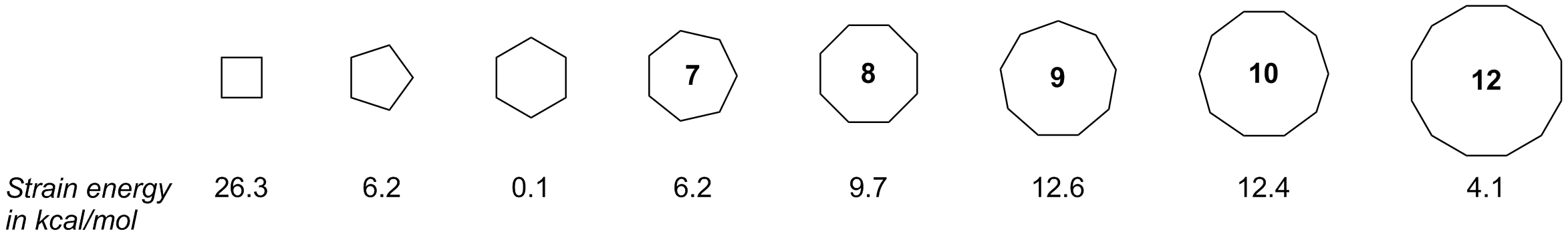
Additional information : Cyclopropane has the highest angle strain among the cycloalkanes, followed by cyclobutane. The bond angle of $s{p_2}$ hybridised carbon is $120^\circ $.
Note: The smaller cycloalkanes have more angle and torsional strains as compared to larger cyclohexane. The more the strains in a molecule the more it is unstable. Angle strain and torsional strains increase potential energy. Cyclobutane and cyclopropane are unstable molecules.
Complete step by step answer:In the given question we have to find which pair of the cycloalkanes have both the torsional and angle strain. Angle strain occurs when there is a deviation of the bond angles from the ideal bond angles. It basically increases the potential energy of the molecule. The smaller cycloalkanes have more torsional strain as compared to the larger cyclohexane. In smaller cycloalkane the hydrogens eclipse each other. In cycloalkane the carbon atom is of $s{p_3}$ hybridisation. The angle of a $s{p_3}$ hybridised atom is $109.5^\circ $ . But in cyclopropane the carbon atoms are maintained in $60^\circ $ . This means that it has an angle strain of $49.5^\circ $ , which is very high. The carbon atoms of the cyclobutene are maintained in $90^\circ $ . The angle strain in cyclobutane is around $19.5^\circ $ . On the other hand, the angle strain of cyclohexane and cyclopentane is negligible. So according to the above explanation it is very much clear to us that cyclopropane and cyclobutane have both torsional and angle strain .
So the correct answer of the above question is :
A: Cyclopropane , Cyclobutane

Additional information : Cyclopropane has the highest angle strain among the cycloalkanes, followed by cyclobutane. The bond angle of $s{p_2}$ hybridised carbon is $120^\circ $.
Note: The smaller cycloalkanes have more angle and torsional strains as compared to larger cyclohexane. The more the strains in a molecule the more it is unstable. Angle strain and torsional strains increase potential energy. Cyclobutane and cyclopropane are unstable molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




