
What is the instantaneous rate of reaction? And how can we determine it?
Answer
558k+ views
Hint:The term instantaneous rate means the reaction rate at a particular time of very short interval. We determine it with the help of plotting the reaction rate vs. time.
Complete step by step answer:
The instantaneous rate of reaction is the rate at some instant at a particular time. Specifically it is defined as the change in concentration of the components of a reaction at an infinitely small time interval. The average of the instantaneous reaction rate over a period of time gives the average rate during the reaction.
For determining the instantaneous rate at a definite time t we need to plot the change in rate of the rate of reaction along the x axis and the time interval along the y axis. Using the plot we calculate the negative of the slope of the curve of the change in concentration of a reactant with respect to the time at time \[t\]. It is done by measuring the slope of the tangent to the graph of concentration vs. time.
Let us consider a reaction in which a reactant \[R\] is converting into a product \[P\]. The reaction is
$R \to P$
If we plot the change in the concentration of the formation of \[P\]with time \[t\], we obtain a curved line instead of a straight line. The plot is as follows:
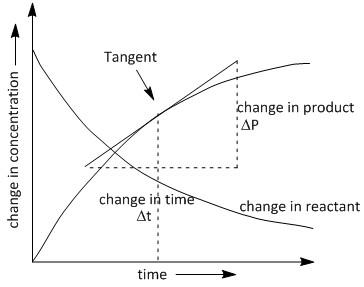
In the above graph we need to draw the best tangent at the time \[t\]where we require the rate of reaction. By extending the endpoints of the tangent along the x and y axis we obtain a triangle where it is convenient to calculate the value of change in concentration of product P with time i.e. $\dfrac{{\Delta P}}{{\Delta t}}$ .
The instantaneous rate of reaction is determined using the slope of the line or the tangent to the curve at any time (\[t\]).
Note:
The rate of concentration of products increases with time according to the plot seen above and the rate of concentration of reactants decreases with time. Thus a negative slope is obtained in plotting the change in concentration of reactant with time.
Complete step by step answer:
The instantaneous rate of reaction is the rate at some instant at a particular time. Specifically it is defined as the change in concentration of the components of a reaction at an infinitely small time interval. The average of the instantaneous reaction rate over a period of time gives the average rate during the reaction.
For determining the instantaneous rate at a definite time t we need to plot the change in rate of the rate of reaction along the x axis and the time interval along the y axis. Using the plot we calculate the negative of the slope of the curve of the change in concentration of a reactant with respect to the time at time \[t\]. It is done by measuring the slope of the tangent to the graph of concentration vs. time.
Let us consider a reaction in which a reactant \[R\] is converting into a product \[P\]. The reaction is
$R \to P$
If we plot the change in the concentration of the formation of \[P\]with time \[t\], we obtain a curved line instead of a straight line. The plot is as follows:

In the above graph we need to draw the best tangent at the time \[t\]where we require the rate of reaction. By extending the endpoints of the tangent along the x and y axis we obtain a triangle where it is convenient to calculate the value of change in concentration of product P with time i.e. $\dfrac{{\Delta P}}{{\Delta t}}$ .
The instantaneous rate of reaction is determined using the slope of the line or the tangent to the curve at any time (\[t\]).
Note:
The rate of concentration of products increases with time according to the plot seen above and the rate of concentration of reactants decreases with time. Thus a negative slope is obtained in plotting the change in concentration of reactant with time.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




