
Is sugar an element, compound or mixture?
Answer
506.7k+ views
Hint: We need to know that the sugar belongs to carbohydrates having different forms. The carbohydrate test is very simple which leads to charing when we burn it on flame. The carbohydrates are easy to test or identify since they give a sweet smell of burning with charring. Glucose, sucrose and fructose are examples for sugar.
Complete answer:
We have to know that an element is a form of matter that is made up of the same kind of atoms, whereas a compound can be derived from the same or different kind of atoms or elements that are chemically joined together. On the other hand mixture is also composed of the same or different kinds of atoms but they are not chemically joined together. Sugar is a compound that is formed by a combination of three atoms: carbon, hydrogen and oxygen. Since these three atoms are chemically joined with each other thus they form a compound in nature. On mixing sugar with water it forms a true solution if it is added in a required amount, if excess sugar is added to water it will lead to saturation.
Chemical structure of glucose can be represented below. Since glucose is a sugar having a molecular formula \[{C_6}{H_{12}}{O_6}\].
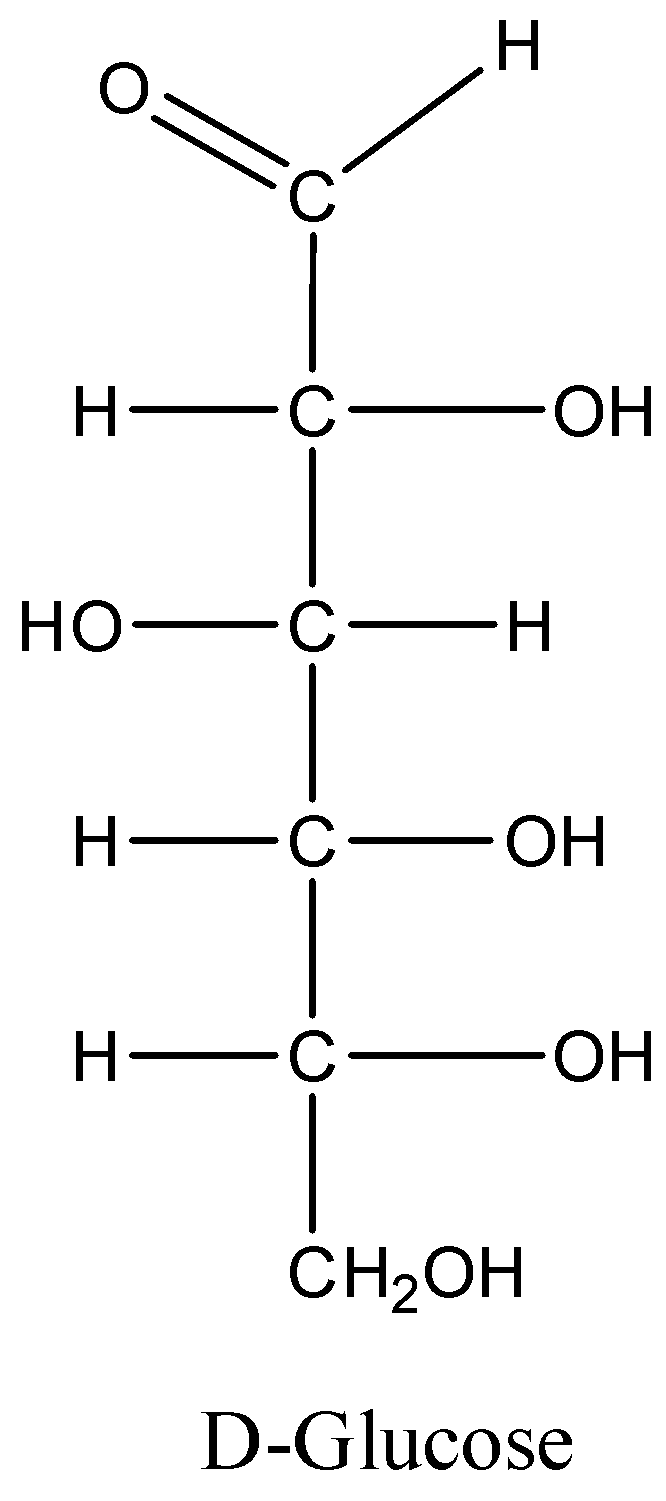
Note:
We have to know that carbohydrates are classified into three classes: monosaccharide, disaccharides and polysaccharides. Monosaccharide is simple sugar containing only one unit of simple sugar i.e. \[{C_6}{H_{12}}{O_6}\] whereas disaccharides contain two units of simple sugar. Glucose is an aldohexose since it has an aldehydic group whereas fructose is a ketohexose since it has a keto group present in it.
Complete answer:
We have to know that an element is a form of matter that is made up of the same kind of atoms, whereas a compound can be derived from the same or different kind of atoms or elements that are chemically joined together. On the other hand mixture is also composed of the same or different kinds of atoms but they are not chemically joined together. Sugar is a compound that is formed by a combination of three atoms: carbon, hydrogen and oxygen. Since these three atoms are chemically joined with each other thus they form a compound in nature. On mixing sugar with water it forms a true solution if it is added in a required amount, if excess sugar is added to water it will lead to saturation.
Chemical structure of glucose can be represented below. Since glucose is a sugar having a molecular formula \[{C_6}{H_{12}}{O_6}\].

Note:
We have to know that carbohydrates are classified into three classes: monosaccharide, disaccharides and polysaccharides. Monosaccharide is simple sugar containing only one unit of simple sugar i.e. \[{C_6}{H_{12}}{O_6}\] whereas disaccharides contain two units of simple sugar. Glucose is an aldohexose since it has an aldehydic group whereas fructose is a ketohexose since it has a keto group present in it.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




