
Isobutyl magnesium bromide with dry ether and absolute alcohol gives:
A.$\
C{H_3} - CH - C{H_2}OH \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,| \\
\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3} \\
\ $ and $C{H_3}C{H_2}MgBr$
B. $\
C{H_3} - CH - C{H_2} - C{H_{3\,}} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,| \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3} \\
\ $ and $Mg(OH)Br$
C. $\
C{H_3} - CH - C{H_3}CH2 - C{H_{2\,}} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,| \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3} \\
\,\,\,\,\,\,\,\,\,\,\,\, \\
\ $and $Mg\left( {OH} \right)Br$
D. $\
C{H_{_3}} - CH - C{H_3} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,| \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3} \\
\ $ and $C{H_3}C{H_2}OMgBr$
Answer
539.4k+ views
Hint: The compounds having a generic formula of $R - Mg - X$ where $X$ is the halogen and $R$ is usually an alkyl or aryl group is called Grignard reagent of Grignard compound. In organic synthesis Grignard reagent is popular in the synthesis of new carbon - carbon bonds.
Complete step by step answer:
In this reaction isobutyl magnesium bromide reacts with absolute alcohol where isobutyl magnesium bromide acts as Grignard reagent and reacts with absolute alcohol. Here Grignard reagents react with the active hydrogen present in absolute alcohol. During this reaction the active hydrogen present in the absolute alcohol gets swapped with $MgBr$ present in isobutyl magnesium bromide forming isobutane and magnesium bromide ethanoate as the end products.
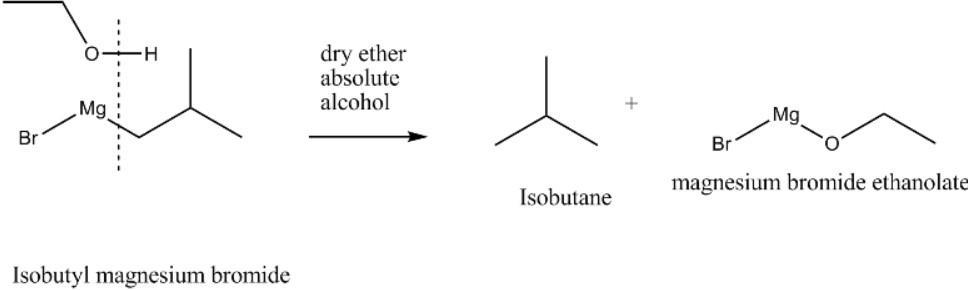
Hence, we get the final products isobutane and magnesium bromide ethanoate.
So, the correct answer is Option D.
Additional information:
Pure Grignard reagents are extremely reactive solids hence they are usually handled as solutions in solvents like diethyl ether or tetrahydrofuran which are stable as long as water is excluded. In those mediums Grignard reagent is invariably present as a complex along with magnesium atoms connected with two ether oxygens by means of coordination bonds.
Note: Grignard reagents are prepared by treating organic handles (normally organo bromine) with magnesium metal. In order to stabilize organo magnesium compounds cyclic or acyclic ethers are used. Water and air rapidly destroys the reagent by protonolysis or oxidation are excluded using air free techniques.
Complete step by step answer:
In this reaction isobutyl magnesium bromide reacts with absolute alcohol where isobutyl magnesium bromide acts as Grignard reagent and reacts with absolute alcohol. Here Grignard reagents react with the active hydrogen present in absolute alcohol. During this reaction the active hydrogen present in the absolute alcohol gets swapped with $MgBr$ present in isobutyl magnesium bromide forming isobutane and magnesium bromide ethanoate as the end products.

Hence, we get the final products isobutane and magnesium bromide ethanoate.
So, the correct answer is Option D.
Additional information:
Pure Grignard reagents are extremely reactive solids hence they are usually handled as solutions in solvents like diethyl ether or tetrahydrofuran which are stable as long as water is excluded. In those mediums Grignard reagent is invariably present as a complex along with magnesium atoms connected with two ether oxygens by means of coordination bonds.
Note: Grignard reagents are prepared by treating organic handles (normally organo bromine) with magnesium metal. In order to stabilize organo magnesium compounds cyclic or acyclic ethers are used. Water and air rapidly destroys the reagent by protonolysis or oxidation are excluded using air free techniques.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




