
How many isomers of $ {C_3}{H_4} $ are possible?
Answer
494.1k+ views
Hint: Molecules having the same molecular formula but have different molecular geometries are known as isomers. It is not necessary that isomers share the same chemical or physical properties. Structural isomerism and stereoisomerism are two types of isomerism, this is further divided into subtypes like geometric, optical stereoisomerism and chain, position, functional, and many more structural isomerism.
Complete Step By Step Answer:
Propyne (an alkyne) has the chemical formula $ C{H_3}C \equiv CH $ and the Degree of unsaturation of $ {C_3}{H_4} $ is 2.
Degree of unsaturation $ = \dfrac{{2 \times Number{\text{ of carbon atom + 2 + }}Number{\text{ of halogens - Number of hydrogen}}}}{2} $
The degree of unsaturation suggests that the isomers will have a $ 2\pi $ bond and presence of a ring with at least $ 1\pi $ bond.
So there are $ 3 $ possible isomers of $ {C_3}{H_4} $ and they are Prop-1-yne, prop-1,2-diene, and cyclopropene.
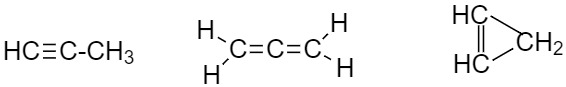
Additional Information:
Chirality is based on the number of groups attached to a carbon atom. The nature of groups i.e. whether it is similar or different is taken into consideration. Chirality is also influenced by the ability to superimpose.
Achiral molecules have a plane of symmetry. If the compounds have mirror images, they are not identical to each other; they are called enantiomers.
Note:
Molecules having the same molecular formula but differ by atoms or bonds are called structural isomers or constitutional isomers but isomers having a difference in the orientation of atoms in space are stereoisomers. Stereoisomers are of two types Geometrical isomer and optical isomer.
Optical isomers also known as Enantiomers are a pair of stereoisomers, related to each other as they are mirror images of each other and are non-superposable. Stereoisomers that are not related through reflection operation are Diastereomers.
Complete Step By Step Answer:
Propyne (an alkyne) has the chemical formula $ C{H_3}C \equiv CH $ and the Degree of unsaturation of $ {C_3}{H_4} $ is 2.
Degree of unsaturation $ = \dfrac{{2 \times Number{\text{ of carbon atom + 2 + }}Number{\text{ of halogens - Number of hydrogen}}}}{2} $
The degree of unsaturation suggests that the isomers will have a $ 2\pi $ bond and presence of a ring with at least $ 1\pi $ bond.
So there are $ 3 $ possible isomers of $ {C_3}{H_4} $ and they are Prop-1-yne, prop-1,2-diene, and cyclopropene.
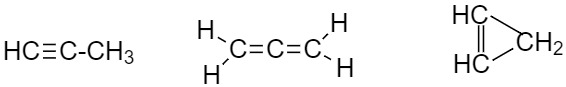
Additional Information:
Chirality is based on the number of groups attached to a carbon atom. The nature of groups i.e. whether it is similar or different is taken into consideration. Chirality is also influenced by the ability to superimpose.
Achiral molecules have a plane of symmetry. If the compounds have mirror images, they are not identical to each other; they are called enantiomers.
Note:
Molecules having the same molecular formula but differ by atoms or bonds are called structural isomers or constitutional isomers but isomers having a difference in the orientation of atoms in space are stereoisomers. Stereoisomers are of two types Geometrical isomer and optical isomer.
Optical isomers also known as Enantiomers are a pair of stereoisomers, related to each other as they are mirror images of each other and are non-superposable. Stereoisomers that are not related through reflection operation are Diastereomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




