
How many isomers of molecular formula ${{C}_{3}}{{H}_{9}}N$ will liberate ${{N}_{2}}$ gas on treatment with $HN{{O}_{2}}$ .
a.) One
b.) Three
c.) Two
d.) Five
Answer
568.5k+ views
Hint: Isomers are defined as the molecules which have the same molecular formula but different molecular geometries. There are two types of isomers such as conformational isomers and constitutional isomers.
Complete Solution :
In the given formulae ${{C}_{3}}{{H}_{9}}N$, four isomers are possible. They are propan-1-amine, propan-2-amine, N-methylethanamine, N,N-Dimethylmethanamine. The structure of different isomers which can be drawn corresponding to the given molecular formula are given below:
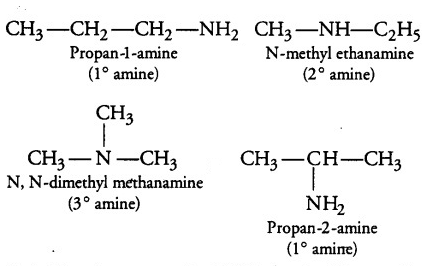
- Among the given isomers of ${{C}_{3}}{{H}_{9}}N$ only primary amines will liberate nitrogen gas when it will be treated with nitrous acid i.e. propan-2-amine and propan-1-amine will liberate nitrogen gas when it will be treated with nitrous acid. The reactions involved are mentioned below:
\[\begin{align}
& \underset{propan-1-amine}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}+HN{{O}_{3}}\to }}\,C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH+{{N}_{2}}+HCl \\
& \underset{propan-2-amine}{\mathop{C{{H}_{3}}CH(N{{H}_{2}})C{{H}_{3}}+HN{{O}_{3}}\to }}\,C{{H}_{3}}CH(OH)C{{H}_{3}}+{{N}_{2}}+HCl \\
\end{align}\]
So, the correct answer is “Option C”.
Additional information:
We know that there is no superimposable mirror image and there is no plane of symmetry in the molecule then the molecule is known as chiral. And the chiral carbon has four different groups attached to the carbon. This property is known as chirality and the compounds which have the same molecular formula but different compounds are said to be isomers. The compounds which are mirror images but are not identical to each other. They are known as enantiomers.
Note: Structural isomers are defined as compounds which have the same molecular formula but different connectivity of atoms or bonds. The isomers which are different by the orientation of atoms in space are known as stereoisomers and the isomers which differ by their rotation around a single bond are known as conformational isomers.
Complete Solution :
In the given formulae ${{C}_{3}}{{H}_{9}}N$, four isomers are possible. They are propan-1-amine, propan-2-amine, N-methylethanamine, N,N-Dimethylmethanamine. The structure of different isomers which can be drawn corresponding to the given molecular formula are given below:

- Among the given isomers of ${{C}_{3}}{{H}_{9}}N$ only primary amines will liberate nitrogen gas when it will be treated with nitrous acid i.e. propan-2-amine and propan-1-amine will liberate nitrogen gas when it will be treated with nitrous acid. The reactions involved are mentioned below:
\[\begin{align}
& \underset{propan-1-amine}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}+HN{{O}_{3}}\to }}\,C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH+{{N}_{2}}+HCl \\
& \underset{propan-2-amine}{\mathop{C{{H}_{3}}CH(N{{H}_{2}})C{{H}_{3}}+HN{{O}_{3}}\to }}\,C{{H}_{3}}CH(OH)C{{H}_{3}}+{{N}_{2}}+HCl \\
\end{align}\]
So, the correct answer is “Option C”.
Additional information:
We know that there is no superimposable mirror image and there is no plane of symmetry in the molecule then the molecule is known as chiral. And the chiral carbon has four different groups attached to the carbon. This property is known as chirality and the compounds which have the same molecular formula but different compounds are said to be isomers. The compounds which are mirror images but are not identical to each other. They are known as enantiomers.
Note: Structural isomers are defined as compounds which have the same molecular formula but different connectivity of atoms or bonds. The isomers which are different by the orientation of atoms in space are known as stereoisomers and the isomers which differ by their rotation around a single bond are known as conformational isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




