
IUPAC name of
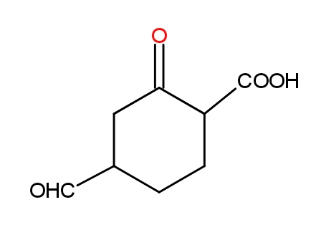
a.) 6-oxo-4-formyl cyclohexane carboxylic acid
b.) 4-Formyl-2-oxo cyclohexane carboxylic acid
c.) 4-Formyl-2-oxo cyclohexanoic acid
d.) 1-Carboxy-4-formyl-2-oxo cyclohexane

Answer
576.6k+ views
Hint: Firstly, find the longest chain and then number carbons such that all substituents get the lowest number possible. Further, the C=O group gas prefix oxo and CHO has formyl. The COOH is carboxylic acid and is named as oic acid. The carbon chain has six carbons. So, the word root is hexane.
Complete step by step answer :
The IUPAC stands for International Union of Pure and Applied Chemistry. It has formulated some rules for the systematic nomenclature of organic compounds which are even revised later on for the uniform naming of compounds. The IUPAC has laid down some rules which govern the naming of all the organic compounds. This is necessary so that there is uniform nomenclature to be followed world-wide. The rules to find the IUPAC name of above compound are -
The first thing one should do is to find the longest carbon chain in the molecule.
The longest chain is a cyclohexane ring. The next thing is to find out all the substituents present on the carbon chain. The substituent with higher priority is numbered 1 and then other substituents. The numbering would be as-
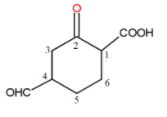
Now, the nomenclature- The C=O group gas prefix oxo and CHO has formyl. The COOH is carboxylic acid and is named as oic acid. The carbon chain has six carbons. So, the word root is hexane. So, the IUPAC name of the compound is - 4-Formyl-2-oxo cyclohexane carboxylic acid
So, the option b.) is the correct option.
Note: It must be noted that the substituent should get the maximum lowest number possible. This is in accordance with the lowest locant rule. Further, the IUPAC nomenclature is needed because earlier every chemist gave its own new name to the molecule it discovered. As a result, one molecule had different names at different places. It was difficult to study them. No one got to know whether the molecule discovered by him/her is the new one or the one that has already been discovered. So, the scientist devised some formulations to uniform the naming of compounds.
Complete step by step answer :
The IUPAC stands for International Union of Pure and Applied Chemistry. It has formulated some rules for the systematic nomenclature of organic compounds which are even revised later on for the uniform naming of compounds. The IUPAC has laid down some rules which govern the naming of all the organic compounds. This is necessary so that there is uniform nomenclature to be followed world-wide. The rules to find the IUPAC name of above compound are -
The first thing one should do is to find the longest carbon chain in the molecule.
The longest chain is a cyclohexane ring. The next thing is to find out all the substituents present on the carbon chain. The substituent with higher priority is numbered 1 and then other substituents. The numbering would be as-

Now, the nomenclature- The C=O group gas prefix oxo and CHO has formyl. The COOH is carboxylic acid and is named as oic acid. The carbon chain has six carbons. So, the word root is hexane. So, the IUPAC name of the compound is - 4-Formyl-2-oxo cyclohexane carboxylic acid
So, the option b.) is the correct option.
Note: It must be noted that the substituent should get the maximum lowest number possible. This is in accordance with the lowest locant rule. Further, the IUPAC nomenclature is needed because earlier every chemist gave its own new name to the molecule it discovered. As a result, one molecule had different names at different places. It was difficult to study them. No one got to know whether the molecule discovered by him/her is the new one or the one that has already been discovered. So, the scientist devised some formulations to uniform the naming of compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




