
Keto-enol tautomerism is observed in:
\[
A.{\text{ }}{C_6}{H_5} - CHO \\
B.{\text{ }}{C_6}{H_5} - CO - C{H_3} \\
C.{\text{ }}{C_6}{H_5} - CO - {C_6}{H_5} \\
D.{\text{ }}{C_6}{H_5} - CO - C{\left( {C{H_3}} \right)_2}{C_6}{H_5} \\
\]
Answer
587.4k+ views
Hint: In order to solve the given problem we will first understand the meaning of tautomerism and different forms of tautomerism. Further we will see the characteristics required for an organic compound to show keto-enol tautomerism and then on the basis of the structure of each of the chemical compounds we will find out the correct options amongst the given ones.
Complete step by step answer:
First let us understand the meaning of isomerism and tautomerism.
Isomerism is a process in which more than one substance has different chemical compositions, but the same chemical formula. Chemical compounds that have similar chemical compositions that vary in properties are called isomers and the arrangement of atoms in the molecule.
Tautomerism is a condition in which two or more interconvertible structures appear to occur in a single chemical product that is distinct in terms of the relative location of one atomic nucleus, which is normally hydrogen.
Keto-enol tautomerism means that the compound will undergo tautomerism and will show the structure of both ketone group and enol group but will have the same chemical formula.
It should be noted that the chemical compound showing keto-enol tautomerism must have alpha $\left( \alpha \right)$ hydrogen in its structure.
So let us first visualize the structure of each of the chemical compounds and check for alpha $\left( \alpha \right)$ hydrogen in the structure.
Option A.
The structure of \[{C_6}{H_5} - CHO\] is:
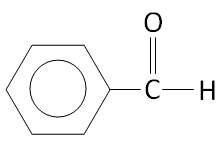
We can see that there is no alpha hydrogen in the structure.
Option B.
The structure of \[{C_6}{H_5} - CO - C{H_3}\] is:
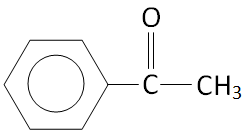
We can see that there are three alpha hydrogen in the structure.
Option C.
The structure of \[{C_6}{H_5} - CO - {C_6}{H_5}\] is:
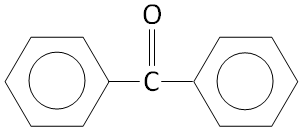
We can see that there is no alpha hydrogen in the structure.
Option D.
The structure of \[{C_6}{H_5} - CO - C{\left( {C{H_3}} \right)_2}{C_6}{H_5}\] is:
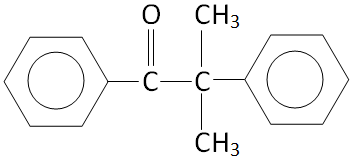
We can see that there is no alpha hydrogen in the structure.
As we know that keto-enol tautomerism is observed only when the compound has alpha hydrogen in the compound so the compound \[{C_6}{H_5} - CO - C{H_3}\] will show keto-enol tautomerism in the following way.
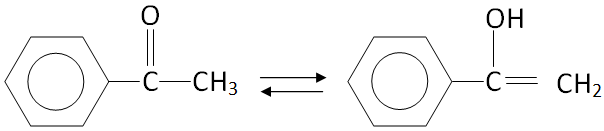
In the above diagram the first structure is ketone and the second structure is alcohol.
Hence, keto-enol tautomerism is observed in \[{C_6}{H_5} - CO - C{H_3}\] .
So, the correct answer is “Option B”.
Note: In order to solve such types of problems students must draw the diagram of the given compounds as it is easier to solve the problems of isomerism by the help of structural diagrams. Students also must know the method to identify the alpha hydrogen and alpha carbon. The carbon atom attached directly to the carbon atom involved in the double bond formation with oxygen is called alpha carbon and the hydrogen atom directly attached to the alpha carbon is alpha hydrogen.
Complete step by step answer:
First let us understand the meaning of isomerism and tautomerism.
Isomerism is a process in which more than one substance has different chemical compositions, but the same chemical formula. Chemical compounds that have similar chemical compositions that vary in properties are called isomers and the arrangement of atoms in the molecule.
Tautomerism is a condition in which two or more interconvertible structures appear to occur in a single chemical product that is distinct in terms of the relative location of one atomic nucleus, which is normally hydrogen.
Keto-enol tautomerism means that the compound will undergo tautomerism and will show the structure of both ketone group and enol group but will have the same chemical formula.
It should be noted that the chemical compound showing keto-enol tautomerism must have alpha $\left( \alpha \right)$ hydrogen in its structure.
So let us first visualize the structure of each of the chemical compounds and check for alpha $\left( \alpha \right)$ hydrogen in the structure.
Option A.
The structure of \[{C_6}{H_5} - CHO\] is:

We can see that there is no alpha hydrogen in the structure.
Option B.
The structure of \[{C_6}{H_5} - CO - C{H_3}\] is:

We can see that there are three alpha hydrogen in the structure.
Option C.
The structure of \[{C_6}{H_5} - CO - {C_6}{H_5}\] is:

We can see that there is no alpha hydrogen in the structure.
Option D.
The structure of \[{C_6}{H_5} - CO - C{\left( {C{H_3}} \right)_2}{C_6}{H_5}\] is:

We can see that there is no alpha hydrogen in the structure.
As we know that keto-enol tautomerism is observed only when the compound has alpha hydrogen in the compound so the compound \[{C_6}{H_5} - CO - C{H_3}\] will show keto-enol tautomerism in the following way.

In the above diagram the first structure is ketone and the second structure is alcohol.
Hence, keto-enol tautomerism is observed in \[{C_6}{H_5} - CO - C{H_3}\] .
So, the correct answer is “Option B”.
Note: In order to solve such types of problems students must draw the diagram of the given compounds as it is easier to solve the problems of isomerism by the help of structural diagrams. Students also must know the method to identify the alpha hydrogen and alpha carbon. The carbon atom attached directly to the carbon atom involved in the double bond formation with oxygen is called alpha carbon and the hydrogen atom directly attached to the alpha carbon is alpha hydrogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




