
What Is the meaning of primary, secondary and tertiary carbon atoms?
Answer
513k+ views
Hint :Catenation is the bonding of atoms of the same element into a series, known as a chain, in chemistry. An open-chain compound is one in which the ends of a chain or ring are not linked to each other, whereas a closed-chain compound is one in which the ends are joined in a ring (a cyclic compound). The terms catenate and catenation come from the Latin word catena, which means "chain."
Complete Step By Step Answer:
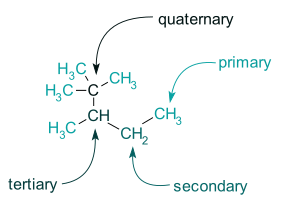
Carbon, which makes covalent connections with other carbon atoms to build larger chains and structures, is the most easily catenated element. The occurrence of such a large number of organic compounds in nature is due to this. Catenation is one of carbon's most well-known characteristics, and organic chemistry is essentially the study of catenated carbon compounds (and known as catenae). In biochemistry, carbon chains incorporate different additional elements onto the backbone of carbon, such as hydrogen, oxygen, and biometals. Carbon, on the other hand, is far from the only element capable of creating such catenae; numerous other main-group elements may also generate a diverse spectrum of catenae. Organic chemists studying carbon chemistry have come up with a variety of abbreviations to express structures and occurrences that would normally take two sentences to explain. Primary, secondary, tertiary, and quaternary are the terms for carbon-containing functional groups. Carbons that are connected to another carbon are referred to as primary carbons. (Hydrogens are neglected in this nomenclature, despite the fact that they are generally three in number in this situation, as we will see.)
Secondary carbons are carbon atoms that are connected to two other carbon atoms.
Three additional carbons are connected to tertiary carbons.
Finally, four additional carbons are joined to form quaternary carbons.
Note :
The electronegativity of the element in issue, the molecular orbital n, and the capacity to form different kinds of covalent bonds are all steric and electronic aspects that impact catenation ability. The sigma overlap between nearby carbon atoms is sufficiently great to allow the formation of fully stable chains. In the past, despite abundance of evidence to the contrary, this was assumed to be exceedingly difficult with additional factors.
Complete Step By Step Answer:
Carbon, which makes covalent connections with other carbon atoms to build larger chains and structures, is the most easily catenated element. The occurrence of such a large number of organic compounds in nature is due to this. Catenation is one of carbon's most well-known characteristics, and organic chemistry is essentially the study of catenated carbon compounds (and known as catenae). In biochemistry, carbon chains incorporate different additional elements onto the backbone of carbon, such as hydrogen, oxygen, and biometals. Carbon, on the other hand, is far from the only element capable of creating such catenae; numerous other main-group elements may also generate a diverse spectrum of catenae. Organic chemists studying carbon chemistry have come up with a variety of abbreviations to express structures and occurrences that would normally take two sentences to explain. Primary, secondary, tertiary, and quaternary are the terms for carbon-containing functional groups. Carbons that are connected to another carbon are referred to as primary carbons. (Hydrogens are neglected in this nomenclature, despite the fact that they are generally three in number in this situation, as we will see.)
Secondary carbons are carbon atoms that are connected to two other carbon atoms.
Three additional carbons are connected to tertiary carbons.
Finally, four additional carbons are joined to form quaternary carbons.

Note :
The electronegativity of the element in issue, the molecular orbital n, and the capacity to form different kinds of covalent bonds are all steric and electronic aspects that impact catenation ability. The sigma overlap between nearby carbon atoms is sufficiently great to allow the formation of fully stable chains. In the past, despite abundance of evidence to the contrary, this was assumed to be exceedingly difficult with additional factors.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




