
What is the mechanism of halogenations of alkanes?
Answer
588k+ views
Hint: Halogenation of alkanes means the substitution of a halogen atom(s) by the removal of one or more hydrogen atoms in the alkane. The mechanism of halogenations occurs in three steps: chain initiation, chain propagation, and chain termination.
Complete step by step answer:
When alkane is treated with a suitable halogen in the presence of ultraviolet light or by heating the reaction mixture to 520-670 K, haloalkane is produced.
For example, chlorination of methane. The reaction is given below:
$\underset{methane}{\mathop C{{H}_{4}}}\,+C{{l}_{2}}\xrightarrow{hv}\underset{chloromethane}{\mathop C{{H}_{3}}Cl}\,+HCl$
$\underset{chloromethane}{\mathop C{{H}_{3}}Cl}\,+C{{l}_{2}}\xrightarrow{hv}\underset{dichloromethane}{\mathop C{{H}_{2}}C{{l}_{2}}}\,+HCl$
$\underset{dichloromethane}{\mathop C{{H}_{2}}C{{l}_{2}}}\,+C{{l}_{2}}\xrightarrow{hv}\underset{trichloromethane}{\mathop CHC{{l}_{3}}}\,+HCl$
$\underset{trichloromethane}{\mathop CHC{{l}_{3}}}\,+C{{l}_{2}}\xrightarrow{hv}\underset{tetrachloromethane}{\mathop CC{{l}_{4}}}\,+HCl$
Mechanism of halogenations: The halogenations occur in three steps and it follows a free-radical mechanism.
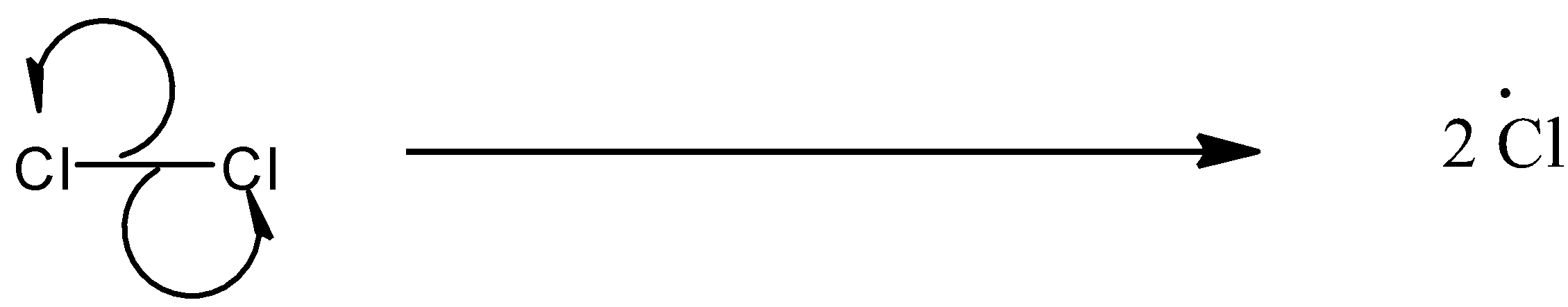
(a)- Chain initiation: When a mixture of $C{{H}_{4}}$and $C{{l}_{2}}$ is heated at 520-670 K in dark or is subjected to UV light at room temperature, $C{{l}_{2}}$ absorbs energy and undergoes homolytic fission which produces chlorine-free radicals.
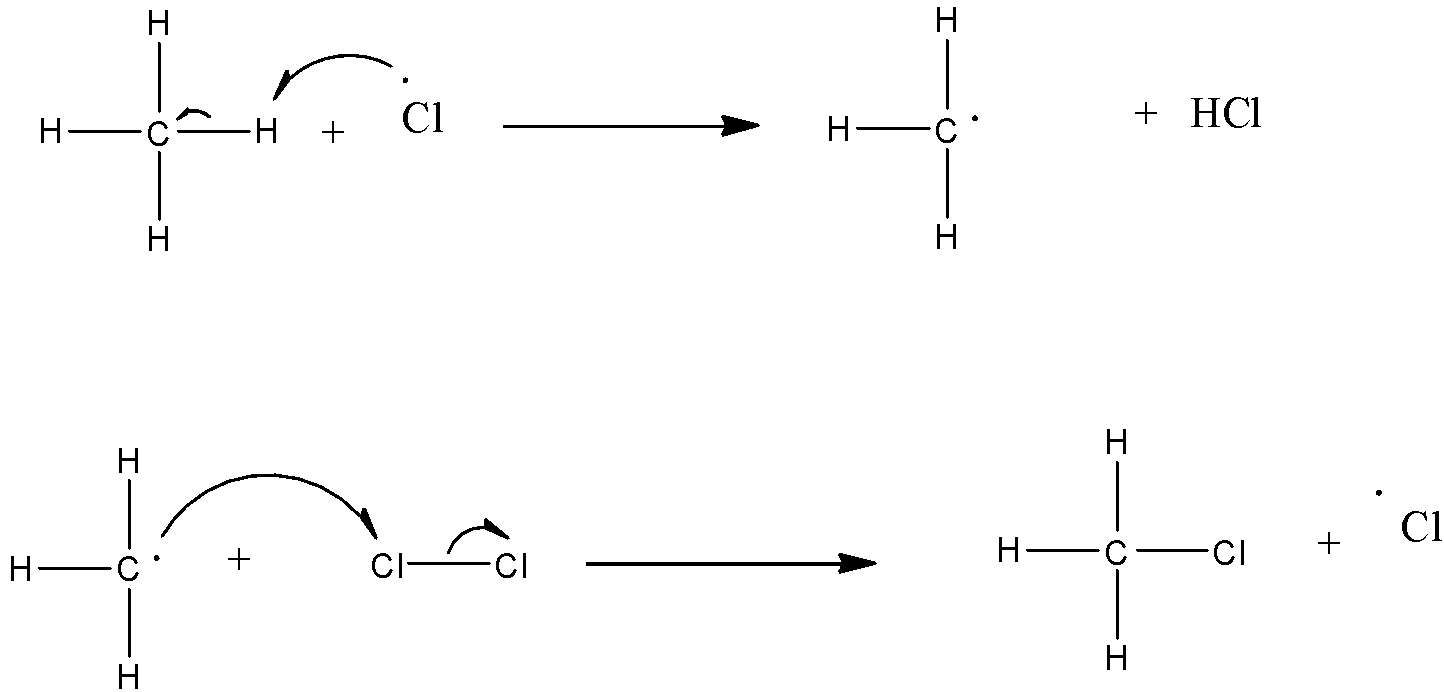
(b)- Chain propagation: There are two steps in propagation. In the first reaction, the $\bullet Cl$ attacks the $C{{H}_{4}}$ molecule and abstracts a hydrogen atom forming $\bullet C{{H}_{3}}$ and a molecule of $HCl$as shown in reaction. In the second reaction, $\bullet C{{H}_{3}}$ thus produced reacts further with a molecule of $C{{l}_{2}}$ forming a molecule of methyl chloride and another $\bullet Cl$. These reactions continue until the formation of $CC{{l}_{4}}$.
$C{{H}_{3}}Cl+\bullet Cl\to \bullet C{{H}_{2}}Cl+HCl$
$\bullet C{{H}_{2}}Cl+C{{l}_{2}}\to C{{H}_{2}}C{{l}_{2}}+\bullet Cl$
$C{{H}_{2}}C{{l}_{2}}+\bullet Cl\to \bullet CHC{{l}_{2}}+HCl$
$\bullet CHC{{l}_{2}}+C{{l}_{2}}\to CHC{{l}_{3}}+\bullet Cl$
$CHC{{l}_{3}}+\bullet Cl\to \bullet CC{{l}_{3}}+HCl$
$\bullet CC{{l}_{3}}+C{{l}_{2}}\to CC{{l}_{4}}+\bullet Cl$
(c)- Chain termination: The chain reactions till now formed to have two types of free radicals combine to form molecules. The reaction is given below:
$\bullet Cl+\bullet Cl\to Cl-Cl$
$\bullet C{{H}_{3}}+\bullet C{{H}_{3}}\to C{{H}_{3}}-C{{H}_{3}}$
$\bullet C{{H}_{3}}+\bullet Cl\to C{{H}_{3}}-Cl$
So, by following these steps the halogenations of an alkane are done.
Note: The order of reactivity of the halogens towards the halogenations reaction of alkanes as follows: ${{F}_{2}}$ > $C{{l}_{2}}$ > $B{{r}_{2}}$ > ${{I}_{2}}$.The iodination reaction is reversible as follows:
$C{{H}_{4}}+{{I}_{2}}\rightleftharpoons C{{H}_{3}}-I+HI$.
Complete step by step answer:
When alkane is treated with a suitable halogen in the presence of ultraviolet light or by heating the reaction mixture to 520-670 K, haloalkane is produced.
For example, chlorination of methane. The reaction is given below:
$\underset{methane}{\mathop C{{H}_{4}}}\,+C{{l}_{2}}\xrightarrow{hv}\underset{chloromethane}{\mathop C{{H}_{3}}Cl}\,+HCl$
$\underset{chloromethane}{\mathop C{{H}_{3}}Cl}\,+C{{l}_{2}}\xrightarrow{hv}\underset{dichloromethane}{\mathop C{{H}_{2}}C{{l}_{2}}}\,+HCl$
$\underset{dichloromethane}{\mathop C{{H}_{2}}C{{l}_{2}}}\,+C{{l}_{2}}\xrightarrow{hv}\underset{trichloromethane}{\mathop CHC{{l}_{3}}}\,+HCl$
$\underset{trichloromethane}{\mathop CHC{{l}_{3}}}\,+C{{l}_{2}}\xrightarrow{hv}\underset{tetrachloromethane}{\mathop CC{{l}_{4}}}\,+HCl$
Mechanism of halogenations: The halogenations occur in three steps and it follows a free-radical mechanism.
(a)- Chain initiation: When a mixture of $C{{H}_{4}}$and $C{{l}_{2}}$ is heated at 520-670 K in dark or is subjected to UV light at room temperature, $C{{l}_{2}}$ absorbs energy and undergoes homolytic fission which produces chlorine-free radicals.

(b)- Chain propagation: There are two steps in propagation. In the first reaction, the $\bullet Cl$ attacks the $C{{H}_{4}}$ molecule and abstracts a hydrogen atom forming $\bullet C{{H}_{3}}$ and a molecule of $HCl$as shown in reaction. In the second reaction, $\bullet C{{H}_{3}}$ thus produced reacts further with a molecule of $C{{l}_{2}}$ forming a molecule of methyl chloride and another $\bullet Cl$. These reactions continue until the formation of $CC{{l}_{4}}$.

$C{{H}_{3}}Cl+\bullet Cl\to \bullet C{{H}_{2}}Cl+HCl$
$\bullet C{{H}_{2}}Cl+C{{l}_{2}}\to C{{H}_{2}}C{{l}_{2}}+\bullet Cl$
$C{{H}_{2}}C{{l}_{2}}+\bullet Cl\to \bullet CHC{{l}_{2}}+HCl$
$\bullet CHC{{l}_{2}}+C{{l}_{2}}\to CHC{{l}_{3}}+\bullet Cl$
$CHC{{l}_{3}}+\bullet Cl\to \bullet CC{{l}_{3}}+HCl$
$\bullet CC{{l}_{3}}+C{{l}_{2}}\to CC{{l}_{4}}+\bullet Cl$
(c)- Chain termination: The chain reactions till now formed to have two types of free radicals combine to form molecules. The reaction is given below:
$\bullet Cl+\bullet Cl\to Cl-Cl$
$\bullet C{{H}_{3}}+\bullet C{{H}_{3}}\to C{{H}_{3}}-C{{H}_{3}}$
$\bullet C{{H}_{3}}+\bullet Cl\to C{{H}_{3}}-Cl$
So, by following these steps the halogenations of an alkane are done.
Note: The order of reactivity of the halogens towards the halogenations reaction of alkanes as follows: ${{F}_{2}}$ > $C{{l}_{2}}$ > $B{{r}_{2}}$ > ${{I}_{2}}$.The iodination reaction is reversible as follows:
$C{{H}_{4}}+{{I}_{2}}\rightleftharpoons C{{H}_{3}}-I+HI$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




