
Mention the uses of silicones.
Answer
592.8k+ views
Hint: Silicones have high resistivity to chemical and thermal energy, low thermal conductivity, resistant to oxygen and UV, very low reactivity, etc. these properties make it useful in very large number of applications.
Complete answer:
-Silicone is a type of polymer also known as polysiloxanes. Its monomer is siloxane which has a formula of: $ - {R_2}Si - O - Si{R_2} - $, where R is an organic group. It basically is a chain of alternating oxygen and silicon which is combined with carbon and hydrogen (alkyl group).
Its structure can be represented as:
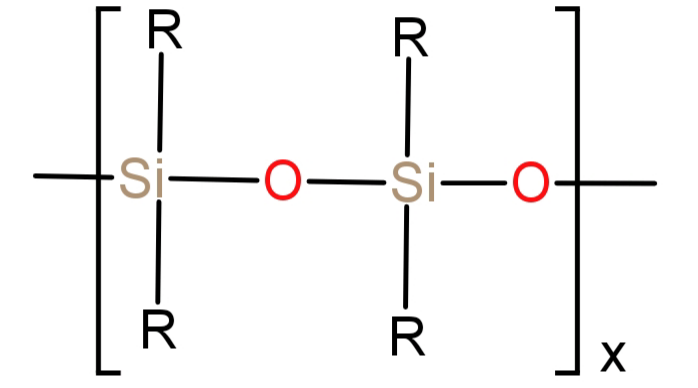
Its general formula can be written as: ${({R_2}SiO)_x}$
-Silicones are quite stable since they have low thermal conductivity, low chemical reactivity and thermal stability. They do not support any sort of microbiological growth, have low toxicity, ability to repel water; is resistant to ozone, UV and oxygen, etc.
-Applications of silicones are:
1) Due to low thermal conductivity it is used in electrical insulation and electronic coatings.
2) The cross linked polymers of silicones are used as non-stick coatings for pans, in paints and in varnish. These paints form the exterior coatings of bridges, houses, railway tracks, etc.
3) Silicone rubber can retain its elasticity and resist chemical attack so used to make seal packing utensils.
4) Silicone fluids made from straight chain polymers of silicone are used to manufacture waterproofing textiles as lubricants and in textiles.
5) Silicone coatings are non-volatile and remain stable even on heating and hence are used for high temperature oil baths, high vacuum pumps, etc.
6) Since they can withstand large stress and even extreme temperatures, silicone sealants and adhesives are used for protecting and sealing doors, windows, wings, hydraulic switches, fuel tanks, wing edges, vent ducts, engine gaskets, etc in the aviation industry.
7) They are used in the construction and renovation of residential buildings.
8) They are also used in making diving masks and goggles because they seal out water well.
9) They increase the efficiency, performance and durability of solar panels and photovoltaic devices and thus make them more cost effective. They are used here because of their thermal resistance.
10) Silicone grease is used as lubricant for brake components in automotive fields.
11) Silicone has many medicinal uses like in breast implants, testicle implants, scar treatment sheets, pectoral implants, etc.
Note: Silicone is often confused with silicon, but they are two different substances. Silicon is a crystalline chemical element and also a semiconducting metalloid used in making integrated circuits, while silicone is made of elements like silicon, carbon, oxygen and hydrogen. Both of them have very different physical and chemical properties.
Complete answer:
-Silicone is a type of polymer also known as polysiloxanes. Its monomer is siloxane which has a formula of: $ - {R_2}Si - O - Si{R_2} - $, where R is an organic group. It basically is a chain of alternating oxygen and silicon which is combined with carbon and hydrogen (alkyl group).
Its structure can be represented as:

Its general formula can be written as: ${({R_2}SiO)_x}$
-Silicones are quite stable since they have low thermal conductivity, low chemical reactivity and thermal stability. They do not support any sort of microbiological growth, have low toxicity, ability to repel water; is resistant to ozone, UV and oxygen, etc.
-Applications of silicones are:
1) Due to low thermal conductivity it is used in electrical insulation and electronic coatings.
2) The cross linked polymers of silicones are used as non-stick coatings for pans, in paints and in varnish. These paints form the exterior coatings of bridges, houses, railway tracks, etc.
3) Silicone rubber can retain its elasticity and resist chemical attack so used to make seal packing utensils.
4) Silicone fluids made from straight chain polymers of silicone are used to manufacture waterproofing textiles as lubricants and in textiles.
5) Silicone coatings are non-volatile and remain stable even on heating and hence are used for high temperature oil baths, high vacuum pumps, etc.
6) Since they can withstand large stress and even extreme temperatures, silicone sealants and adhesives are used for protecting and sealing doors, windows, wings, hydraulic switches, fuel tanks, wing edges, vent ducts, engine gaskets, etc in the aviation industry.
7) They are used in the construction and renovation of residential buildings.
8) They are also used in making diving masks and goggles because they seal out water well.
9) They increase the efficiency, performance and durability of solar panels and photovoltaic devices and thus make them more cost effective. They are used here because of their thermal resistance.
10) Silicone grease is used as lubricant for brake components in automotive fields.
11) Silicone has many medicinal uses like in breast implants, testicle implants, scar treatment sheets, pectoral implants, etc.
Note: Silicone is often confused with silicon, but they are two different substances. Silicon is a crystalline chemical element and also a semiconducting metalloid used in making integrated circuits, while silicone is made of elements like silicon, carbon, oxygen and hydrogen. Both of them have very different physical and chemical properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




