
How many metamers of formula \[{C_4}{H_{10}}O\] are possible?
A.\[2\]
B.\[3\]
C.\[4\]
D.\[1\]
Answer
512.4k+ views
Hint: Metamerism is a type of isomerism in which compounds having the same molecular formula but they possess different alkyl groups on either side of the functional group. The compounds which possess this type of isomerism are called metamers.
Complete answer:
In this question we have to find the metamer of a given compound i.e. \[{C_4}{H_{10}}O\]
We know that metamerism is a type of structural isomerism in which the compounds have the same molecular formula but the arrangement of alkyl groups is different on either side of the bridging functional group. The compounds which show this type of isomerism are termed as metamers.
Some of the functional groups which show metamerism are (\[ - o - \],\[ - S - \], \[ - NH - \], \[ - C( = O) - \], esters, amides etc.)
In other words, Metamers have the same functional group as well as the same molecular formula but they have different structural formulas.
So, the given compound has a molecular formula \[{C_4}{H_{10}}O\] which indicates that it is an ether. It has a functional group (\[ - o - \]). The metamers of these compounds possess different alkyl groups on either side of the oxygen atom.
Here, the possible metamers of the given compound are as follows:-
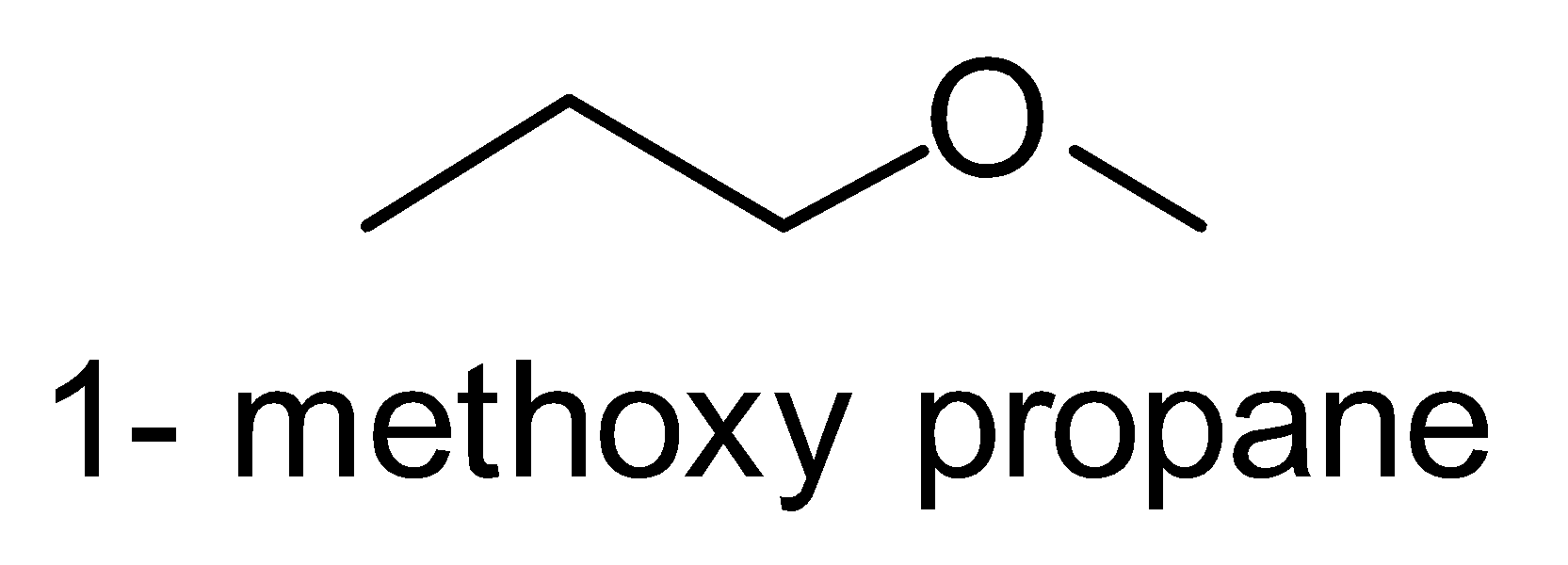
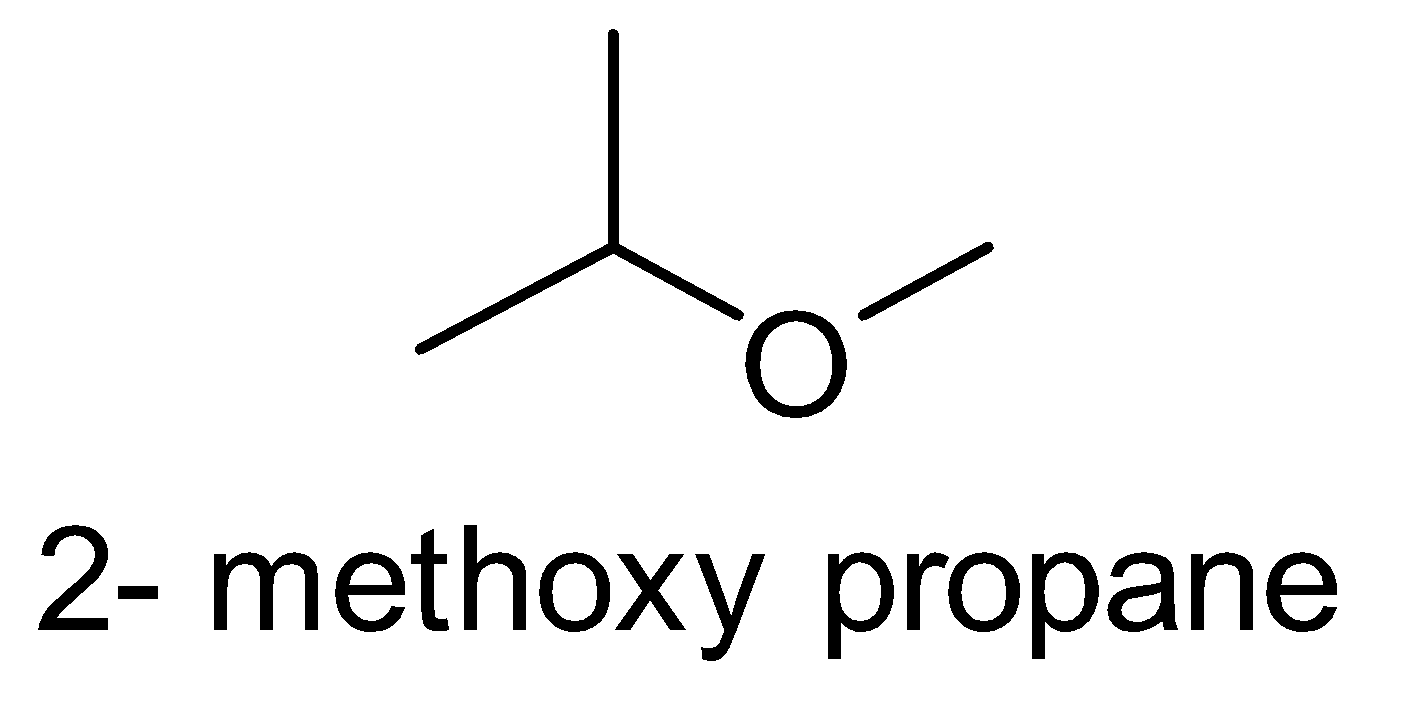
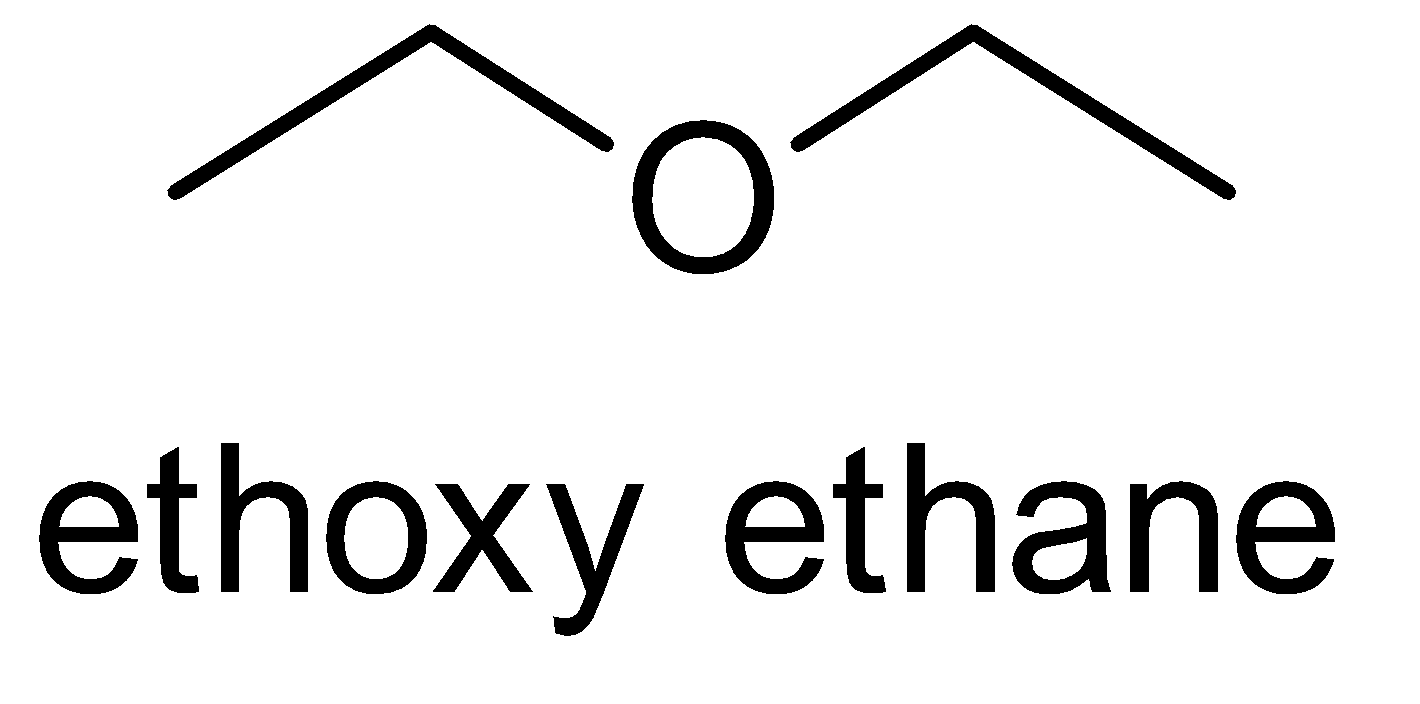
There are only three possibilities of structural isomerism of this compound. So, the given compound \[{C_4}{H_{10}}O\] has three possible metamers.
The correct answer is option (B).
Note:
Metamerism is a rare type of isomerism and it is limited to the compound that contains a functional group which is divalent in nature such as oxygen and sulphur surrounded by alkyl groups. You must remember that it is one of the types of structural isomerism that occurs when the functional groups are the same.
Complete answer:
In this question we have to find the metamer of a given compound i.e. \[{C_4}{H_{10}}O\]
We know that metamerism is a type of structural isomerism in which the compounds have the same molecular formula but the arrangement of alkyl groups is different on either side of the bridging functional group. The compounds which show this type of isomerism are termed as metamers.
Some of the functional groups which show metamerism are (\[ - o - \],\[ - S - \], \[ - NH - \], \[ - C( = O) - \], esters, amides etc.)
In other words, Metamers have the same functional group as well as the same molecular formula but they have different structural formulas.
So, the given compound has a molecular formula \[{C_4}{H_{10}}O\] which indicates that it is an ether. It has a functional group (\[ - o - \]). The metamers of these compounds possess different alkyl groups on either side of the oxygen atom.
Here, the possible metamers of the given compound are as follows:-



There are only three possibilities of structural isomerism of this compound. So, the given compound \[{C_4}{H_{10}}O\] has three possible metamers.
The correct answer is option (B).
Note:
Metamerism is a rare type of isomerism and it is limited to the compound that contains a functional group which is divalent in nature such as oxygen and sulphur surrounded by alkyl groups. You must remember that it is one of the types of structural isomerism that occurs when the functional groups are the same.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




