
Methyl alcohol when reacted with $HI$ forms methyl iodide as a product.
$A.$ True
$B.$ False
Answer
553.8k+ views
Hint: Methyl alcohol when reacting with $HI$ it undergoes a substitution reaction ( i.e. alcohol $\left( { - OH} \right)$ group is substituted by iodide). The reaction proceeds through the $S{N^2}$ mechanism.
Complete answer:
As we know it is a substitution reaction in which alcohol group of methyl alcohol is substituted by iodide of $HI$ and forms methyl iodide as a product. Here is the mechanism of this reaction, Methyl alcohol reacts to form alkyl halides under acidic conditions by an $S{N^2}$ mechanism.
In this reaction the function of $HI$ acid is to produce a protonated alcohol. The halide ions$({I^ - })$ then displace a molecule of water from carbon, this produces a methyl iodide.
Here is the reaction involved in this mechanism,
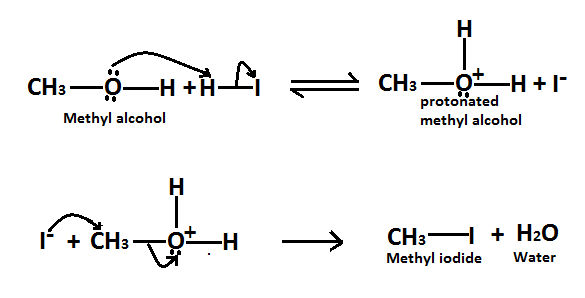
Therefore it is true when methyl alcohol reacts with $HI$ to form methyl iodide as a product. So the correct option is $A.$
Additional information: As we see above methyl iodide are prepared by treatment of methyl alcohol with hydroiodic acid. It can also be prepared by heating alcohols with sodium or potassium iodide in $95\% $ phosphoric acid. The reaction given below represents this process.
$C{H_3}OH + KI\xrightarrow{{95\% {H_3}P{O_3}}}C{H_3}I + KI$
Methyl alcohol: Methyl alcohol is also known as methanol, the chemical formula of methyl alcohol is $\left( {C{H_3}OH} \right)$ . It is light, volatile and flammable liquid.
Note: It is to be noted that secondary and tertiary alcohol react with methyl iodide to form alkyl halides by $S{N^1}$ reaction with protonated alcohol acting as the substrate whereas the primary alcohol react with methyl alcohol to alkyl halides by $S{N^2}$ reaction. The order of reactivity of alcohol with halide is as follows tertiary alcohol react faster than secondary alcohol and secondary alcohol react faster than primary alcohols
The order of reactivity of alcohols is ${3^ \circ } > {2^ \circ } > {1^ \circ }$ .
Complete answer:
As we know it is a substitution reaction in which alcohol group of methyl alcohol is substituted by iodide of $HI$ and forms methyl iodide as a product. Here is the mechanism of this reaction, Methyl alcohol reacts to form alkyl halides under acidic conditions by an $S{N^2}$ mechanism.
In this reaction the function of $HI$ acid is to produce a protonated alcohol. The halide ions$({I^ - })$ then displace a molecule of water from carbon, this produces a methyl iodide.
Here is the reaction involved in this mechanism,

Therefore it is true when methyl alcohol reacts with $HI$ to form methyl iodide as a product. So the correct option is $A.$
Additional information: As we see above methyl iodide are prepared by treatment of methyl alcohol with hydroiodic acid. It can also be prepared by heating alcohols with sodium or potassium iodide in $95\% $ phosphoric acid. The reaction given below represents this process.
$C{H_3}OH + KI\xrightarrow{{95\% {H_3}P{O_3}}}C{H_3}I + KI$
Methyl alcohol: Methyl alcohol is also known as methanol, the chemical formula of methyl alcohol is $\left( {C{H_3}OH} \right)$ . It is light, volatile and flammable liquid.
Note: It is to be noted that secondary and tertiary alcohol react with methyl iodide to form alkyl halides by $S{N^1}$ reaction with protonated alcohol acting as the substrate whereas the primary alcohol react with methyl alcohol to alkyl halides by $S{N^2}$ reaction. The order of reactivity of alcohol with halide is as follows tertiary alcohol react faster than secondary alcohol and secondary alcohol react faster than primary alcohols
The order of reactivity of alcohols is ${3^ \circ } > {2^ \circ } > {1^ \circ }$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




