
What is the molecular mass of cane sugar?
Answer
510.3k+ views
Hint: Sucrose is the chemical name for cane sugar. The sugar molecule in cane is made up of two monosaccharides, glucose and fructose. \[{C_{12}}{H_{22}}{O_{11}}\] is the chemical formula for this non-reducing disaccharide. Table sugar is another name for cane sugar.
Complete answer:
Sucrose is a kind of sugar that is widely used. It's a disaccharide, which means it's made up of two monosaccharides: glucose and fructose. Sucrose is a naturally occurring sugar that is refined into table sugar. \[{C_{12}}{H_{22}}{O_{11}}\] is its molecular formula.
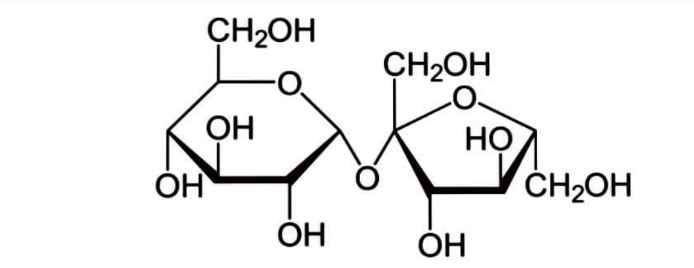
Sucrose is harvested and refined from either sugarcane or sugar beet for human use. Sugar mills crush sugarcane and generate raw sugar, which is delivered to other industries for refining into pure sucrose. They are often located in tropical locations near where sugarcane is farmed. Sugar beet factories are found in temperate locations where the beet is farmed, and they convert the beets into refined sugar. The raw sugar crystals are washed before being dissolved in a sugar syrup, which is then filtered before being run over charcoal to remove any remaining colour. The sugar syrup is then concentrated by boiling under vacuum and crystallised as the final purification step, yielding transparent, odourless, and sweet crystals of pure sucrose.
Cane sugar (\[{C_{12}}{H_{22}}{O_{11}}\]) has a molecular mass of:
\[ \Rightarrow {\text{ }}12{\text{ }} \times {\text{ }}12{\text{ }} + {\text{ }}1{\text{ }} \times {\text{ }}22{\text{ }} + {\text{ }}16{\text{ }} \times {\text{ }}11 \Rightarrow {\text{ }}144{\text{ }} + {\text{ }}22{\text{ }} + {\text{ }}176 \Rightarrow \;{\mathbf{342}}{\text{ }}{\mathbf{g}}/{\mathbf{mol}}\]
Note:
Sugar is frequently used in the production of food and in the preparation of recipes. In\[2017\], about \[185\] million tonnes of sugar were produced around the world.
Sucrose is especially harmful for tooth decay because Streptococcus mutans bacteria convert it to a sticky, extracellular, dextran-based polysaccharide that allows them to adhere together and produce plaque. The only sugar that bacteria can use to make this sticky polysaccharide is sucrose.
Complete answer:
Sucrose is a kind of sugar that is widely used. It's a disaccharide, which means it's made up of two monosaccharides: glucose and fructose. Sucrose is a naturally occurring sugar that is refined into table sugar. \[{C_{12}}{H_{22}}{O_{11}}\] is its molecular formula.

Sucrose is harvested and refined from either sugarcane or sugar beet for human use. Sugar mills crush sugarcane and generate raw sugar, which is delivered to other industries for refining into pure sucrose. They are often located in tropical locations near where sugarcane is farmed. Sugar beet factories are found in temperate locations where the beet is farmed, and they convert the beets into refined sugar. The raw sugar crystals are washed before being dissolved in a sugar syrup, which is then filtered before being run over charcoal to remove any remaining colour. The sugar syrup is then concentrated by boiling under vacuum and crystallised as the final purification step, yielding transparent, odourless, and sweet crystals of pure sucrose.
Cane sugar (\[{C_{12}}{H_{22}}{O_{11}}\]) has a molecular mass of:
\[ \Rightarrow {\text{ }}12{\text{ }} \times {\text{ }}12{\text{ }} + {\text{ }}1{\text{ }} \times {\text{ }}22{\text{ }} + {\text{ }}16{\text{ }} \times {\text{ }}11 \Rightarrow {\text{ }}144{\text{ }} + {\text{ }}22{\text{ }} + {\text{ }}176 \Rightarrow \;{\mathbf{342}}{\text{ }}{\mathbf{g}}/{\mathbf{mol}}\]
Note:
Sugar is frequently used in the production of food and in the preparation of recipes. In\[2017\], about \[185\] million tonnes of sugar were produced around the world.
Sucrose is especially harmful for tooth decay because Streptococcus mutans bacteria convert it to a sticky, extracellular, dextran-based polysaccharide that allows them to adhere together and produce plaque. The only sugar that bacteria can use to make this sticky polysaccharide is sucrose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




