
What is the most stable conformer for 3-methylpentane, viewed along the ${{\text{C}}_{2}}-{{\text{C}}_{3}}$ bond using the Newman projections?
Answer
526.8k+ views
Hint: In Newman's projection, any particular carbon-carbon bond is viewed along its longitudinal axis. The carbon having a lower number is placed at the front and denoted as a point. While the other carbon is placed at the back and denoted as a circle. The most stable conformer is the one having two bulky groups placed at opposite ends and having minimum steric strain.
Complete answer:
3-methylpentane is a structural isomer of hexane having the chemical formula - $\text{C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{2}}\text{CH}\left( \text{C}{{\text{H}}_{3}} \right)\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{3}}$.
Its molecular structure is –
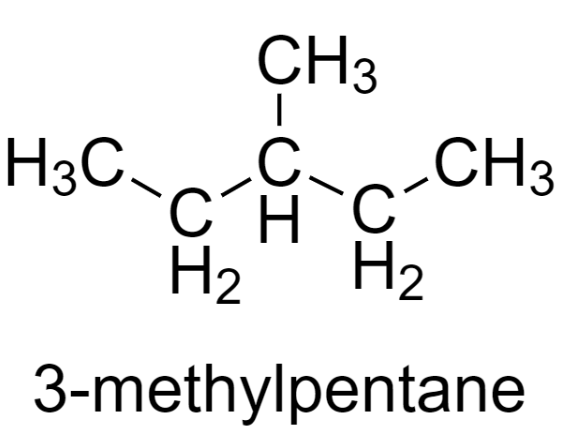
To find the most stable conformer of 3-methylpentane, we need to convert the molecular structure to Newman projection viewed along the ${{\text{C}}_{2}}-{{\text{C}}_{3}}$ bond.
So, the carbon at the front will be ${{\text{C}}_{2}}$ (point) and carbon at the back will be ${{\text{C}}_{3}}$ (circle).
Now, there are many possible conformers of 3-methylpentane, but only a few of them will be stable under the normal set of conditions. The possible stable conformers are drawn below:
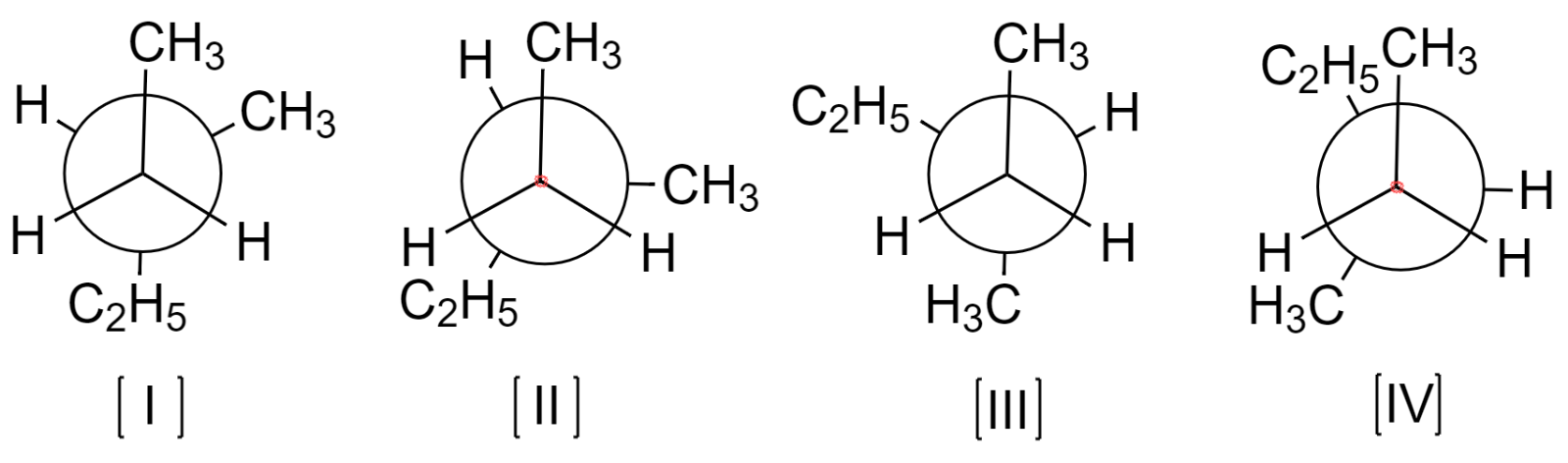
Out of these conformers, the most stable one is that having the bulkier groups far apart from each other. Thus, conformer (I) will be the most stable.
Note:
All the conformers are not very stable at room temperature and are rapidly interconverting to each other. The most stable conformer is said to be the one having bulkier groups away from each other due to the less steric hindrance between such groups.
Complete answer:
3-methylpentane is a structural isomer of hexane having the chemical formula - $\text{C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{2}}\text{CH}\left( \text{C}{{\text{H}}_{3}} \right)\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{3}}$.
Its molecular structure is –

To find the most stable conformer of 3-methylpentane, we need to convert the molecular structure to Newman projection viewed along the ${{\text{C}}_{2}}-{{\text{C}}_{3}}$ bond.
So, the carbon at the front will be ${{\text{C}}_{2}}$ (point) and carbon at the back will be ${{\text{C}}_{3}}$ (circle).
Now, there are many possible conformers of 3-methylpentane, but only a few of them will be stable under the normal set of conditions. The possible stable conformers are drawn below:

Out of these conformers, the most stable one is that having the bulkier groups far apart from each other. Thus, conformer (I) will be the most stable.
Note:
All the conformers are not very stable at room temperature and are rapidly interconverting to each other. The most stable conformer is said to be the one having bulkier groups away from each other due to the less steric hindrance between such groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




