
Name the reagents used to convert phenol into
1.picric acid
2.2,4,6-Tribromophenol
3.Benzene
4.o-phenolsulfonic acid
Answer
573.9k+ views
Hint: Before approaching the reaction, we should know the formulae, of what the reactant is, what the product is needed in the question. Only then our approach to the question will work.
Complete step by step answer:
1.Converting Phenol into picric acid.
Formula of Phenol: \[{C_6}{H_5}OH\]
Formula of picric acid: \[{C_6}{H_3}{N_3}{O_7}\]
Reagent required: concentrated nitric acid\[(HN{O_3})\], concentrated sulphuric acid \[({H_2}S{O_4})\]
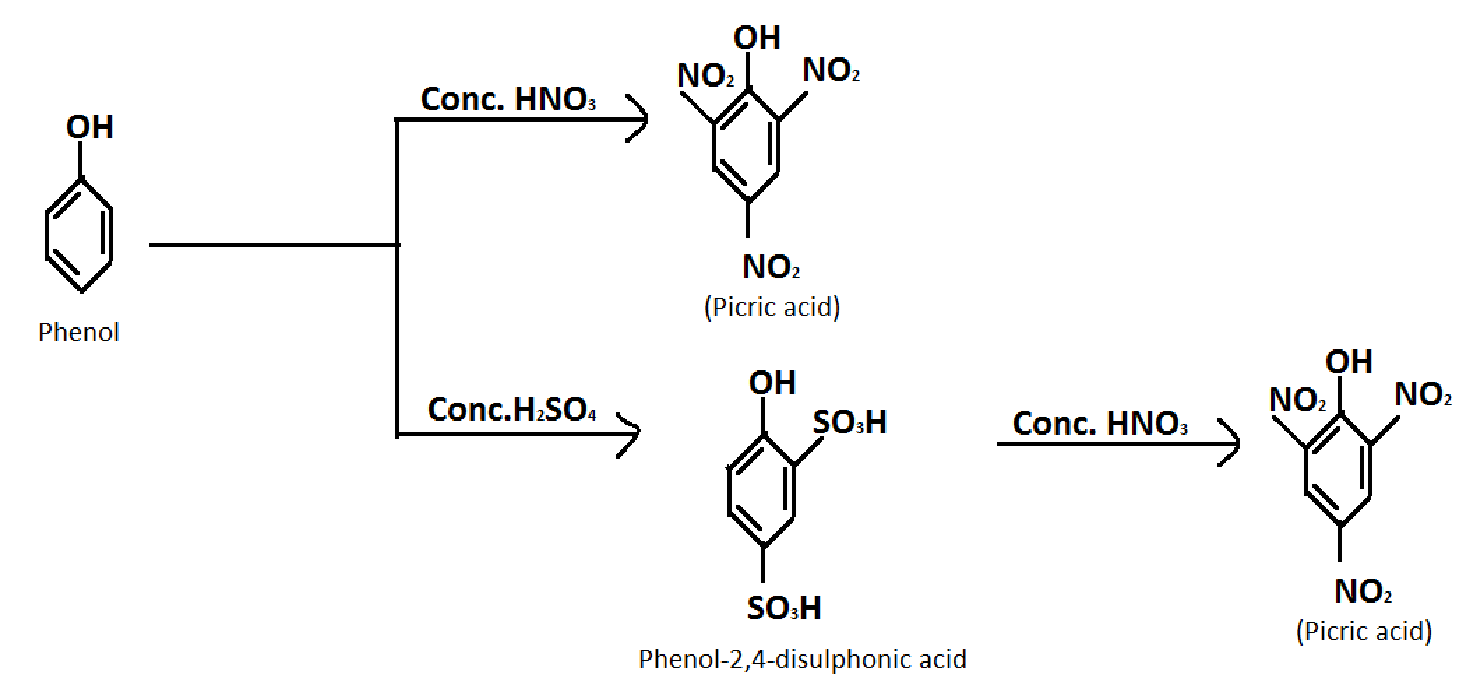
In the first reaction, the yield is poor. So we prefer the second reaction for good yield.
2.Converting phenol into 2,4,6-Tribromophenol
Formula of 2,4,6-Tribromophenol:\[{C_6}{H_3}B{r_3}O\]
Reagent required: Bromine water
Reaction occurs as follows:
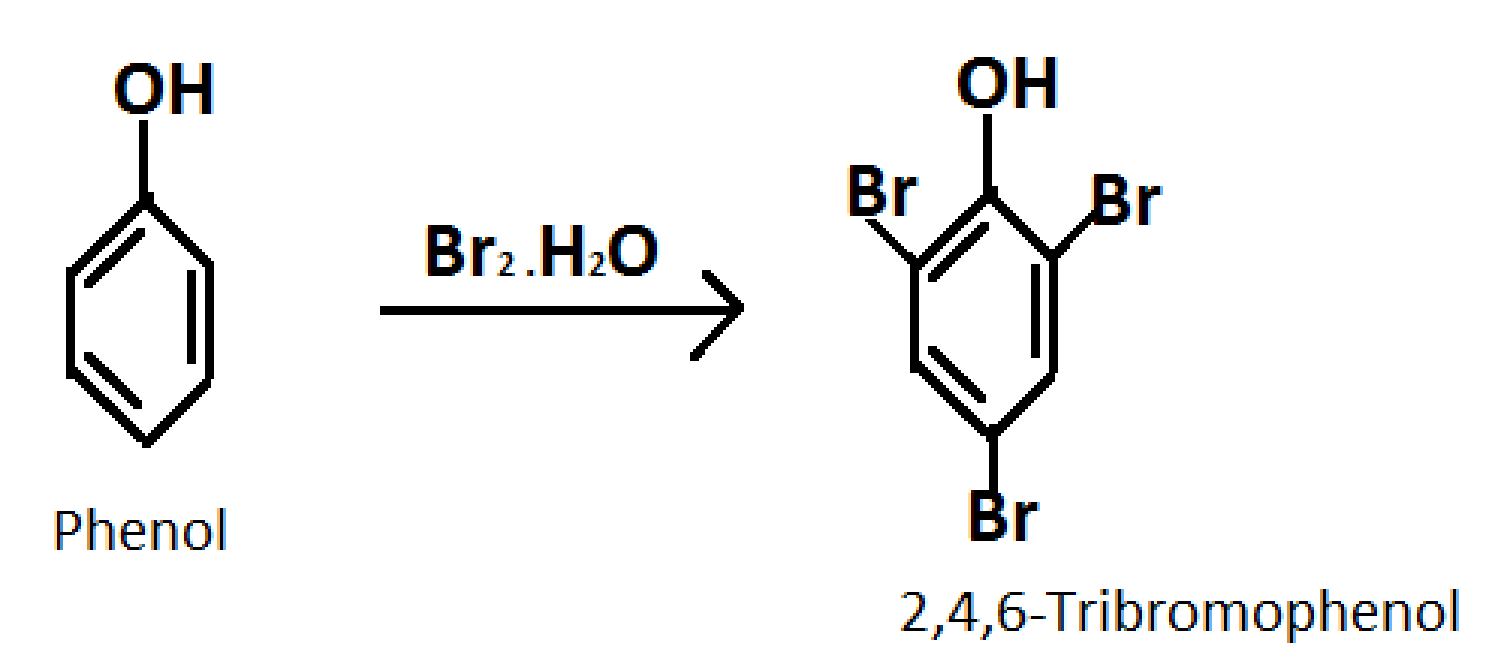
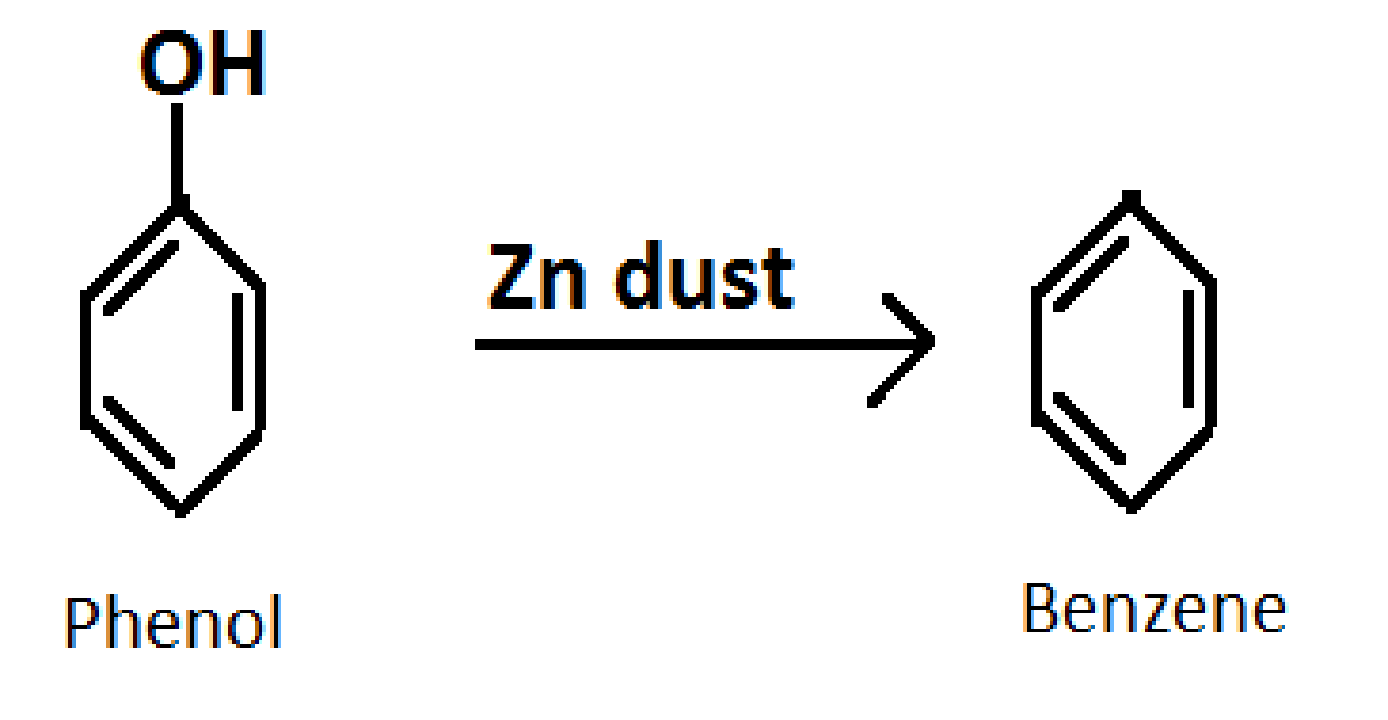
3.Converting phenol into benzene
Formula of Benzene: \[{C_6}{H_6}\]
Reagent required: Zinc \[\left( {Zn} \right)\] dust
Reaction:
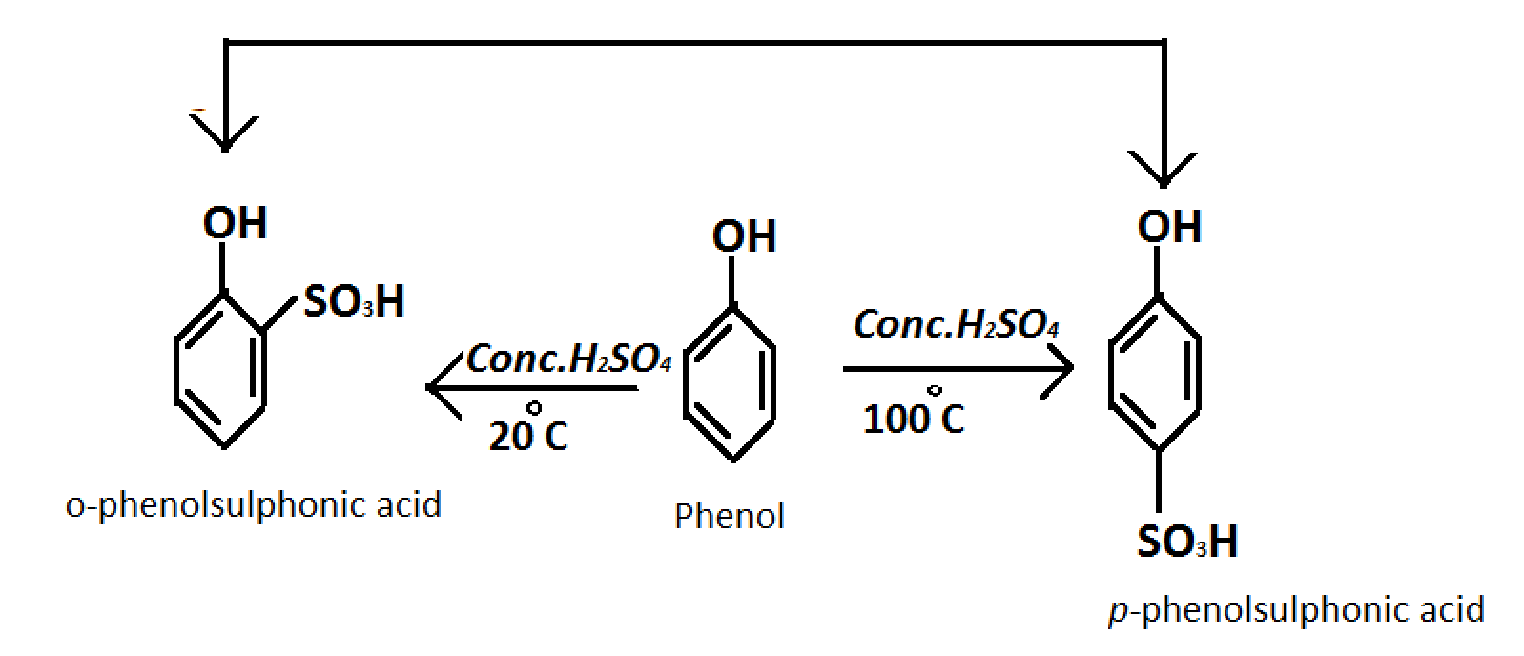
Converting phenol into a-phenolsulfonic acid Formula of Benzene: \[{C_6}{H_6}{O_4}S\]
Reagent required: concentrated sulphuric acid \[({H_2}S{O_4})\] at \[{20^0}C\]
Reaction:
Note:
These are the most important reactions from the exam point of view. The reaction of Converting phenol into 2,4,6-Tribromophenol using bromine water is a lab test for checking the \[ - OH\]functional group. Phenol decolorizes the bromine water and gives us the white precipitates of 2,4,6-Tribromophenol.
Complete step by step answer:
1.Converting Phenol into picric acid.
Formula of Phenol: \[{C_6}{H_5}OH\]
Formula of picric acid: \[{C_6}{H_3}{N_3}{O_7}\]
Reagent required: concentrated nitric acid\[(HN{O_3})\], concentrated sulphuric acid \[({H_2}S{O_4})\]

In the first reaction, the yield is poor. So we prefer the second reaction for good yield.
2.Converting phenol into 2,4,6-Tribromophenol
Formula of 2,4,6-Tribromophenol:\[{C_6}{H_3}B{r_3}O\]
Reagent required: Bromine water
Reaction occurs as follows:

3.Converting phenol into benzene
Formula of Benzene: \[{C_6}{H_6}\]
Reagent required: Zinc \[\left( {Zn} \right)\] dust
Reaction:

Converting phenol into a-phenolsulfonic acid Formula of Benzene: \[{C_6}{H_6}{O_4}S\]
Reagent required: concentrated sulphuric acid \[({H_2}S{O_4})\] at \[{20^0}C\]
Reaction:

Note:
These are the most important reactions from the exam point of view. The reaction of Converting phenol into 2,4,6-Tribromophenol using bromine water is a lab test for checking the \[ - OH\]functional group. Phenol decolorizes the bromine water and gives us the white precipitates of 2,4,6-Tribromophenol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




