
n-Hexane can be converted to benzene by the treatment with:
A. $alc.KMn{O_4}$
B. $alc.KOH$
C. $C{r_2}{O_3}$at 700K
D. $LiAl{H_4}$
Answer
584.7k+ views
Hint: Benzene is an aromatic carbon with 6 carbon atoms. N-Hexane is a straight chain carbon compound with 6 carbon atoms.
Complete step by step answer:
We know that when the n-Hexane molecule reacts with $C{r_2}{O_3}$at 700K, it results in the formation of a benzene compound.
We can write the reaction for this conversion of the n-Hexane compound to the Benzene compound as follows:
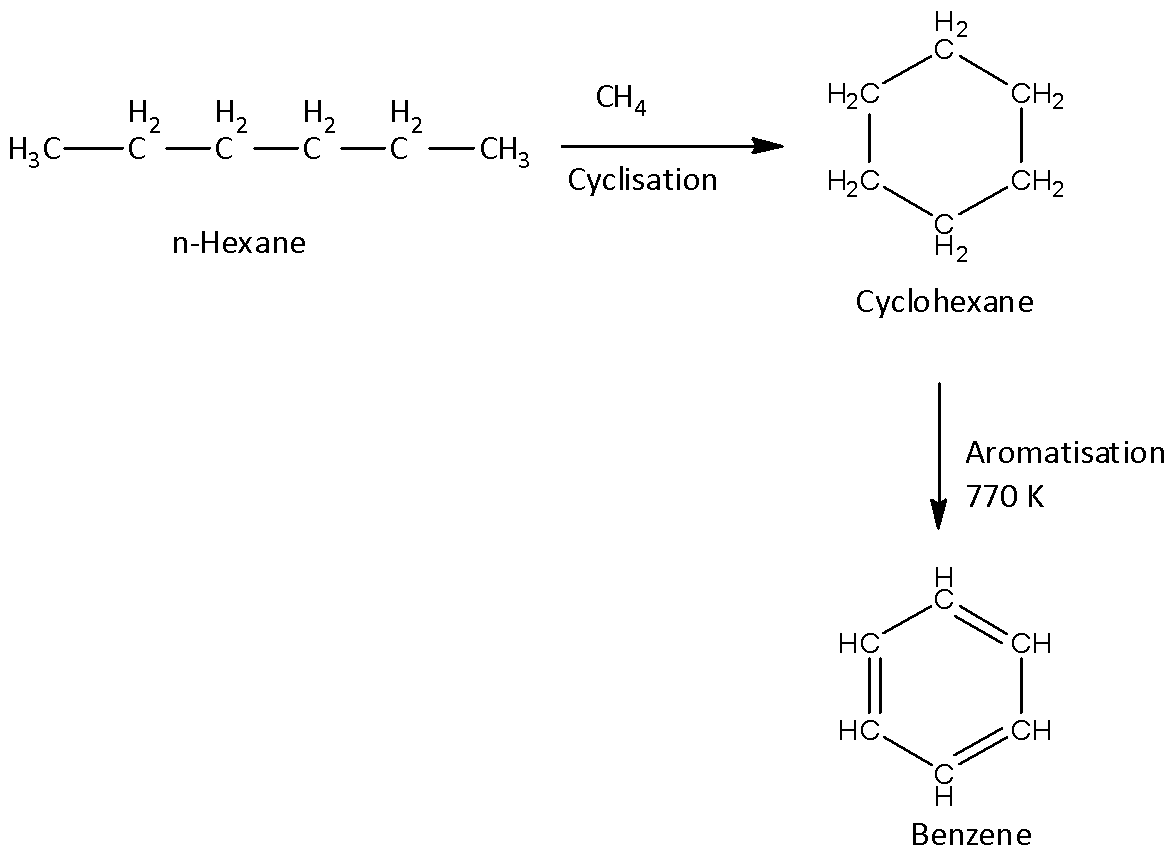
In this reaction, the chromium oxide acts as the catalyst and we can carry out this reaction at high temperature and high pressure. Firstly, the n-Hexane molecule undergoes cyclization to form a cyclohexane compound. This cyclohexane compound undergoes aromatisation in the presence of high temperature of 770 K to form the benzene molecule.
So, the correct answer is Option C .
Additional Information:
We know that a cyclic compound is a compound where atoms are connected or joined in the form of a ring structure. In cyclic compounds, all the connected atoms can be carbon, that is carbocycles, none of the atoms can be carbon which are referred to as inorganic cyclic compounds and atoms can be both carbon and non-carbon which are referred to as heterocyclic compounds. Benzene is considered to be the most stable cyclic compound.
Note:
Benzene has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}$.This compound is soluble in water solution and it is a colourless compound. N-Hexane has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{14}}$.This compound is also water soluble and it is also a colourless compound.
Complete step by step answer:
We know that when the n-Hexane molecule reacts with $C{r_2}{O_3}$at 700K, it results in the formation of a benzene compound.
We can write the reaction for this conversion of the n-Hexane compound to the Benzene compound as follows:

In this reaction, the chromium oxide acts as the catalyst and we can carry out this reaction at high temperature and high pressure. Firstly, the n-Hexane molecule undergoes cyclization to form a cyclohexane compound. This cyclohexane compound undergoes aromatisation in the presence of high temperature of 770 K to form the benzene molecule.
So, the correct answer is Option C .
Additional Information:
We know that a cyclic compound is a compound where atoms are connected or joined in the form of a ring structure. In cyclic compounds, all the connected atoms can be carbon, that is carbocycles, none of the atoms can be carbon which are referred to as inorganic cyclic compounds and atoms can be both carbon and non-carbon which are referred to as heterocyclic compounds. Benzene is considered to be the most stable cyclic compound.
Note:
Benzene has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}$.This compound is soluble in water solution and it is a colourless compound. N-Hexane has the chemical formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{14}}$.This compound is also water soluble and it is also a colourless compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




