
Number of carbons in a ring of deoxyribose sugar is
A. Three
B. Four
C. Five
D. Six
Answer
585k+ views
Hint: Deoxy sugar is derived from the sugar ribose by loss of an oxygen atom. The pentose sugars arabinose and ribose only differ by the stereochemistry at C2′, 2-deoxyribose and 2-deoxy arabinose are equivalent, although the latter term is never used because ribose, not arabinose, is that the precursor to deoxyribose.
Complete answer:
Deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H.
Structure: Several isomers exist with the formula H−(C=O)−(CH2)−(CHOH)3−H, but in deoxyribose, all the hydroxyl groups are on the identical side within the Fischer projection. The term "2-deoxyribose" may confer with either of two enantiomers: the biologically important D-2-deoxyribose and to the rarely encountered reflexion L-2-deoxyribose.[3] D-2-deoxyribose could be a precursor to the supermolecule DNA. 2-deoxyribose is an aldopentose, that is, a monosaccharide with five carbon atoms and having an aldehyde functional group.
In solution, deoxyribose primarily exists as a combination of three structures: the linear form H−(C=O)−(CH2)−(CHOH)3−H and two ring forms, deoxyribofuranose ("C3′-endo"), with a five-membered ring, and deoxyribofuranose ("C2′-endo"), with a six-membered ring. The latter form is predominant (whereas the C3′-endo form is favored for ribose).
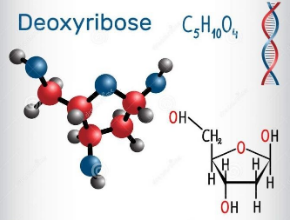
So, the correct answer is option C, five.
Note: Biological importance: As a component of DNA, 2-deoxyribose derivatives have a crucial role in biology.[4] The DNA (deoxyribonucleic acid) molecule, which is that the main repository of genetic information in life, consists of a protracted chain of deoxyribose-containing units called nucleotides, linked via phosphate groups. Within the standard supermolecule nomenclature, a DNA nucleotide consists of a deoxyribose molecule with an organic base (usually adenine, thymine, guanine, or cytosine) attached to the 1′ ribose carbon. The 5′ hydroxyl of every deoxyribose unit is replaced by a phosphate (forming a nucleotide) that's attached to the 3′ carbon of the deoxyribose within the preceding unit.
The absence of the 2′ hydroxyl in deoxyribose is seemingly accountable for the increased mechanical flexibility of DNA compared to RNA, which allows it to assume the double-helix conformation, and also (in the eukaryotes) to be compactly coiled within the little organelle. The double-stranded DNA molecules are typically for much longer than RNA molecules. The backbone of RNA and DNA are structurally similar, but RNA is single-stranded, and made of ribose as opposition deoxyribose.
Other biologically important derivatives of deoxyribose include mono-, di-, and triphosphates, still as 3′-5′ cyclic monophosphates.
Complete answer:
Deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H.
Structure: Several isomers exist with the formula H−(C=O)−(CH2)−(CHOH)3−H, but in deoxyribose, all the hydroxyl groups are on the identical side within the Fischer projection. The term "2-deoxyribose" may confer with either of two enantiomers: the biologically important D-2-deoxyribose and to the rarely encountered reflexion L-2-deoxyribose.[3] D-2-deoxyribose could be a precursor to the supermolecule DNA. 2-deoxyribose is an aldopentose, that is, a monosaccharide with five carbon atoms and having an aldehyde functional group.
In solution, deoxyribose primarily exists as a combination of three structures: the linear form H−(C=O)−(CH2)−(CHOH)3−H and two ring forms, deoxyribofuranose ("C3′-endo"), with a five-membered ring, and deoxyribofuranose ("C2′-endo"), with a six-membered ring. The latter form is predominant (whereas the C3′-endo form is favored for ribose).

So, the correct answer is option C, five.
Note: Biological importance: As a component of DNA, 2-deoxyribose derivatives have a crucial role in biology.[4] The DNA (deoxyribonucleic acid) molecule, which is that the main repository of genetic information in life, consists of a protracted chain of deoxyribose-containing units called nucleotides, linked via phosphate groups. Within the standard supermolecule nomenclature, a DNA nucleotide consists of a deoxyribose molecule with an organic base (usually adenine, thymine, guanine, or cytosine) attached to the 1′ ribose carbon. The 5′ hydroxyl of every deoxyribose unit is replaced by a phosphate (forming a nucleotide) that's attached to the 3′ carbon of the deoxyribose within the preceding unit.
The absence of the 2′ hydroxyl in deoxyribose is seemingly accountable for the increased mechanical flexibility of DNA compared to RNA, which allows it to assume the double-helix conformation, and also (in the eukaryotes) to be compactly coiled within the little organelle. The double-stranded DNA molecules are typically for much longer than RNA molecules. The backbone of RNA and DNA are structurally similar, but RNA is single-stranded, and made of ribose as opposition deoxyribose.
Other biologically important derivatives of deoxyribose include mono-, di-, and triphosphates, still as 3′-5′ cyclic monophosphates.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




