
What is the number of geometrical isomers of the given compound?
\[C{{H}_{3}}CH=CHC{{H}_{2}}CH=C{{H}_{2}}\]
Answer
569.4k+ views
Hint:
-Geometrical and optical isomers are subdivisions of stereoisomerism as it deals with the stereochemistry of the molecule.
-In other words, it takes in account the spatial arrangement of a molecule or ion, as well as the superimposable or non-superimposable characteristics of mirror images.
Complete step by step answer:
The molecules that have the same molecular formula, but the ones who have a different arrangement of the atoms of the molecule in space are termed as Isomers. This definition excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds. The atoms in which making up the numerous isomers are connected in a different order, the phenomena is known as structural isomerism.
In stereoisomerism, the atoms which are making up the isomers are connected in the same order, but they still manage to have a different type of spatial arrangement. Geometric isomerism is a type of stereoisomerism.
The geometrical isomers are those isomers that occur where we have restricted rotation somewhere in a molecule. Some common examples usually include the carbon-carbon double bonds. When two carbons are connected by a double bond, they have restricted rotation as the bond will break if they tried to undergo rotation. Unlike a single bond, where rotation is much easier.
There are two subdivision of geometrical isomerism, cis and trans isomerism.
Trans isomerism is the type where the functional groups are present at different sides of the double bond. And cis isomerism is where the functional group is present at the same side of the double bond.
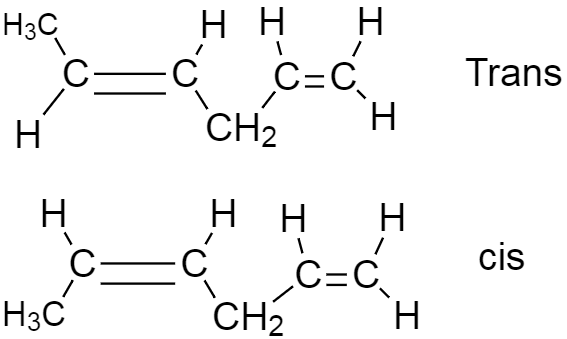
As we can see the double bond in four five positions show geometrical isomerism as it has at least two different functional groups in the two carbons which are attached to double bond but in the, but in the one two position, three of the functional groups are same, so it cannot show isomerism.
Thus the total number of geometrical isomers shown by the given compound are $2$.
Note:Structural isomerism is not a form of stereoisomerism, it is a different type of isomerism and should be confused with stereoisomerism.
-The definition of isomers excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds
-Geometrical and optical isomers are subdivisions of stereoisomerism as it deals with the stereochemistry of the molecule.
-In other words, it takes in account the spatial arrangement of a molecule or ion, as well as the superimposable or non-superimposable characteristics of mirror images.
Complete step by step answer:
The molecules that have the same molecular formula, but the ones who have a different arrangement of the atoms of the molecule in space are termed as Isomers. This definition excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds. The atoms in which making up the numerous isomers are connected in a different order, the phenomena is known as structural isomerism.
In stereoisomerism, the atoms which are making up the isomers are connected in the same order, but they still manage to have a different type of spatial arrangement. Geometric isomerism is a type of stereoisomerism.
The geometrical isomers are those isomers that occur where we have restricted rotation somewhere in a molecule. Some common examples usually include the carbon-carbon double bonds. When two carbons are connected by a double bond, they have restricted rotation as the bond will break if they tried to undergo rotation. Unlike a single bond, where rotation is much easier.
There are two subdivision of geometrical isomerism, cis and trans isomerism.
Trans isomerism is the type where the functional groups are present at different sides of the double bond. And cis isomerism is where the functional group is present at the same side of the double bond.

As we can see the double bond in four five positions show geometrical isomerism as it has at least two different functional groups in the two carbons which are attached to double bond but in the, but in the one two position, three of the functional groups are same, so it cannot show isomerism.
Thus the total number of geometrical isomers shown by the given compound are $2$.
Note:Structural isomerism is not a form of stereoisomerism, it is a different type of isomerism and should be confused with stereoisomerism.
-The definition of isomers excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




