
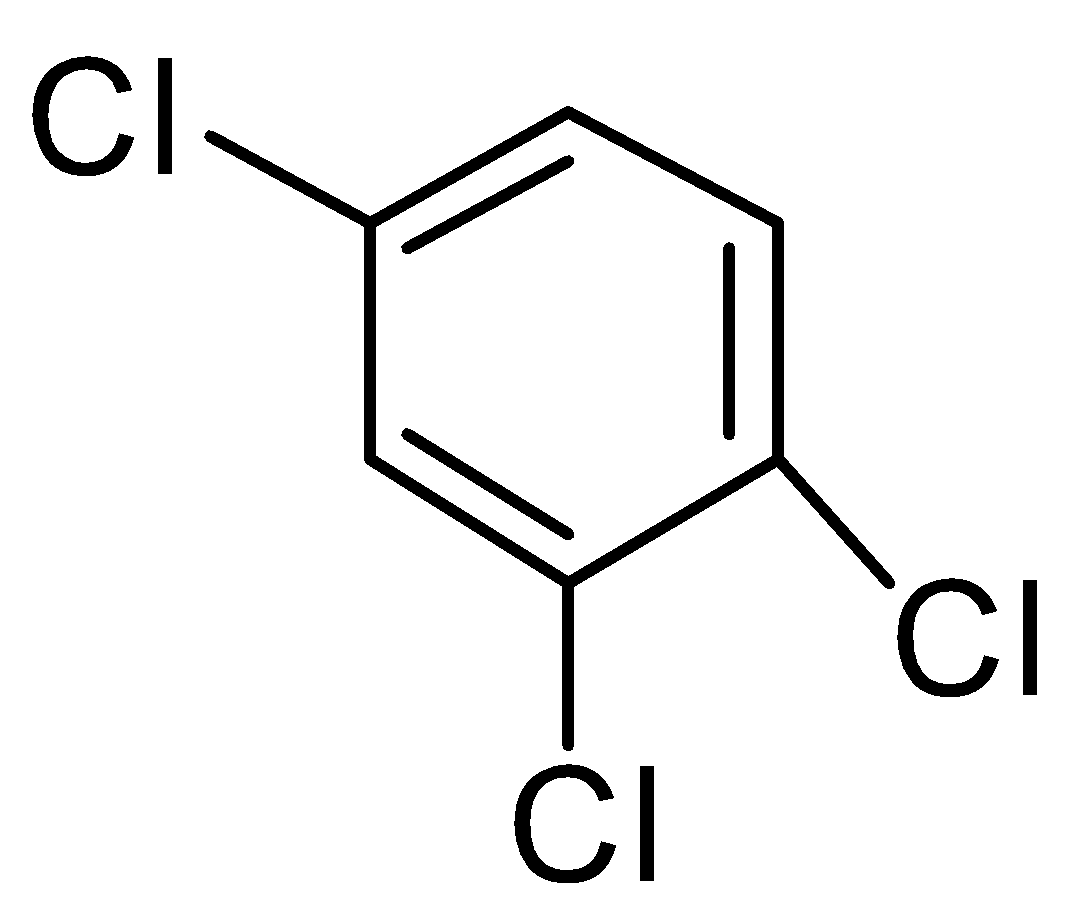
Number of planes of symmetry in the above molecule is:
a.) 0
b.) 1
c.) 2
d.) 3

Answer
570k+ views
Hint: There are three elements of symmetry: Plane of symmetry, point of symmetry and axis of symmetry. If any of these elements are present in a molecule then in spite of having a chiral centre it doesn’t show optical isomerism. A plane of symmetry is an imaginary plane that bisects a molecule into halves that are mirror images of each other.
Complete Solution :
To understand the plane of symmetry let’s take an example:
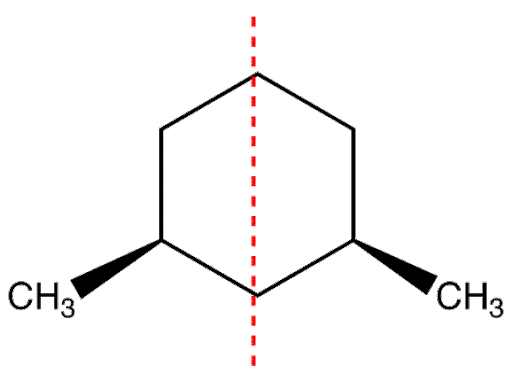
The plane shown in the red line is perpendicular to the plane of the ring and dividing it into two halves are mirror images of each-other.
- The molecule given to us 1,2,4-trichlorobenzene , is a planar molecule.
If we talk about any plane that is perpendicular to its plane then it can’t divide it into two equal halves that are mirror images of each-other.
- Now if we talk a plane that is in the plane of the ring then definitely it will divide it into two equal halves and both will be mirror images of each-other and this plane in the plane of the ring can be drawn by dotted lines as shown below:
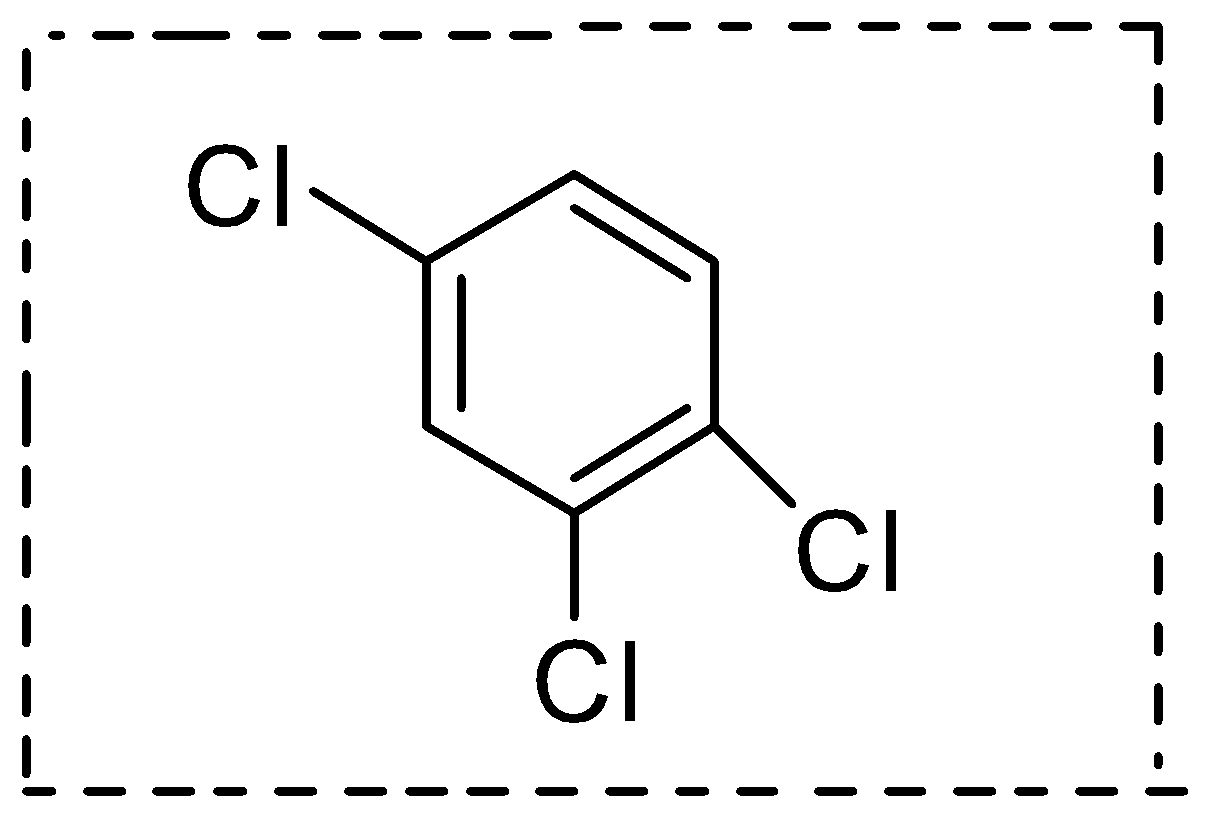
Therefore this molecule has one plane of symmetry.
So, the correct answer is “Option B”.
Additional Information. Every benzene derivative will be having at least one plane of symmetry that will be in the plane of the ring.
Note: 1,2,4-trichlorobenzene appears as colorless liquid at room temperature with a sharp chlorobenzene odor. It is useful as a high-temperature solvent. Aside from its use as a solvent, this compound is a useful precursor to dye and pesticides. Animal studies have shown that 1,2,4-trichlorobenzene affects the liver and kidney, and is possibly a teratogen.
Complete Solution :
To understand the plane of symmetry let’s take an example:

The plane shown in the red line is perpendicular to the plane of the ring and dividing it into two halves are mirror images of each-other.
- The molecule given to us 1,2,4-trichlorobenzene , is a planar molecule.
If we talk about any plane that is perpendicular to its plane then it can’t divide it into two equal halves that are mirror images of each-other.
- Now if we talk a plane that is in the plane of the ring then definitely it will divide it into two equal halves and both will be mirror images of each-other and this plane in the plane of the ring can be drawn by dotted lines as shown below:

Therefore this molecule has one plane of symmetry.
So, the correct answer is “Option B”.
Additional Information. Every benzene derivative will be having at least one plane of symmetry that will be in the plane of the ring.
Note: 1,2,4-trichlorobenzene appears as colorless liquid at room temperature with a sharp chlorobenzene odor. It is useful as a high-temperature solvent. Aside from its use as a solvent, this compound is a useful precursor to dye and pesticides. Animal studies have shown that 1,2,4-trichlorobenzene affects the liver and kidney, and is possibly a teratogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




