
Number of possible position isomers for dichlorobenzene is:
A.Two.
B.Three.
C.Four.
D.Five.
Answer
499.5k+ views
Hint: We know that the position isomer can be defined as the isomer which differs in position of the substituted atom in the chain or ring. Some of the compounds have the same chemical formula but the arrangement of the atoms in the compound is different.
Complete answer:
As we know that the chemical compounds are formed by the difference in the arrangement of the atoms which constitute the compound. These compounds are named isomers. Here we have the structure of the compound given to us have dichloro i.e., two chlorine atoms are there, and a benzene ring is present also, when the same compound has a different spatial arrangement in the space, then that is called stereoisomers, while the isomers which only are differentiated with the position of the substituent group are called positional isomers. The position isomers of dibromo benzene can be drawn as follows:
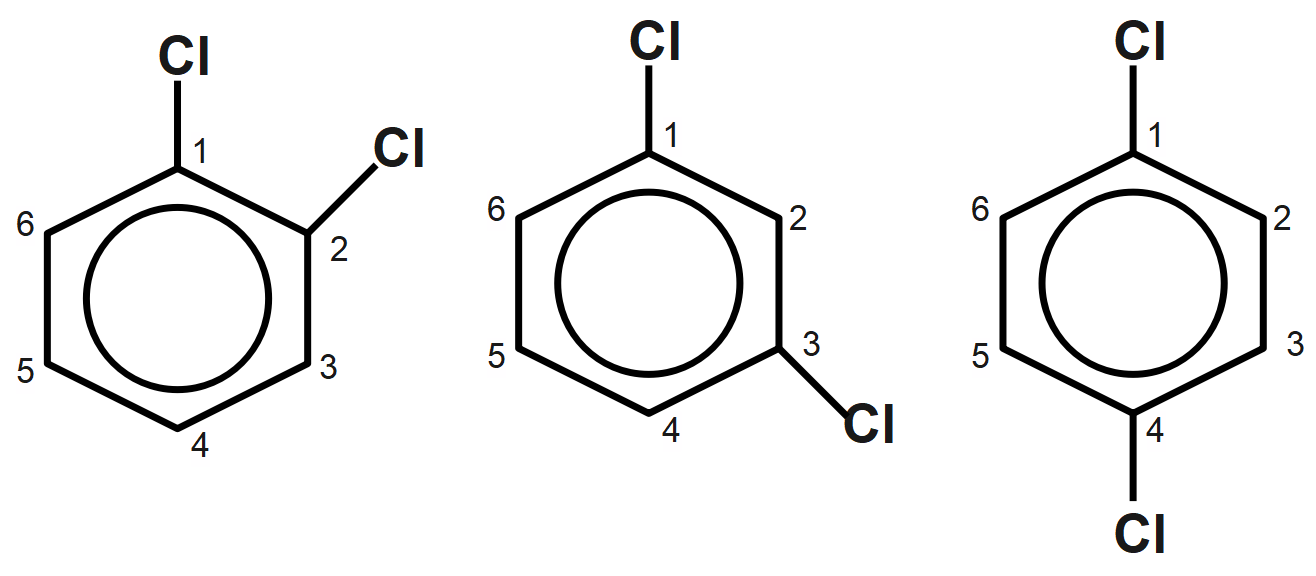
Thus, the positional isomers are the same compounds but the position of the substituents which are attached to the main group will be different in each of the isomeric compounds.
Therefore, the correct answer is option B i.e., the number of possible position isomers for dichlorobenzene is three.
Note:
Remember that the carbon which is attached to only one other carbon atom is called primary carbon, the carbon atom with the attachment of two other carbon atoms is secondary carbon whereas the carbon atom with the attachment of three carbon atoms is termed as a tertiary carbon atom.
Complete answer:
As we know that the chemical compounds are formed by the difference in the arrangement of the atoms which constitute the compound. These compounds are named isomers. Here we have the structure of the compound given to us have dichloro i.e., two chlorine atoms are there, and a benzene ring is present also, when the same compound has a different spatial arrangement in the space, then that is called stereoisomers, while the isomers which only are differentiated with the position of the substituent group are called positional isomers. The position isomers of dibromo benzene can be drawn as follows:

Thus, the positional isomers are the same compounds but the position of the substituents which are attached to the main group will be different in each of the isomeric compounds.
Therefore, the correct answer is option B i.e., the number of possible position isomers for dichlorobenzene is three.
Note:
Remember that the carbon which is attached to only one other carbon atom is called primary carbon, the carbon atom with the attachment of two other carbon atoms is secondary carbon whereas the carbon atom with the attachment of three carbon atoms is termed as a tertiary carbon atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




