
How is Nylon 6,6 obtained. Give reaction
Answer
592.5k+ views
Hint:Nylon 6,6 is a type of polyamide or nylon. It is made up of two monomers each containing 6 carbon atoms, hexamethylenediamine and adipic acid.
Complete step by step answer:
Nylon,6,6 is synthesized by polycondensation of hexamethylenediamine and adipic acid.
Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium/ carbonate mixture and thus molten nylon,6,6 is formed.
To prepare Nylon 6,6 adipic acid and hexamethylenediamine are heated at 553 K under pressure.
This reaction is a shown:
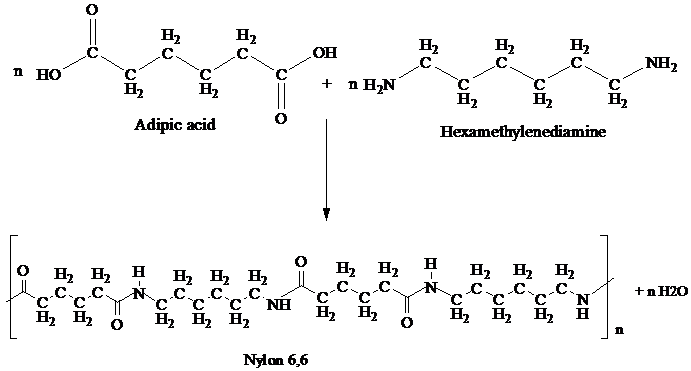
The resulting polymer is extruded into a wide range of fiber types. The fibers are drawn, or sketched in a process that increases their length and reorients the materials molecules parallel to one another to produce a strong, elastic filament.
Note:
Nylon 6,6 has long molecular chains resulting in more hydrogen bonds, creating chemical springs and making it very resilient. It is an amorphous solid so it has a large elastic property and is slightly soluble in boiling water and is very stable in nature. It is very difficult to dye, but one it is dyed it has a high colorfastness and is less susceptible to fading.
Complete step by step answer:
Nylon,6,6 is synthesized by polycondensation of hexamethylenediamine and adipic acid.
Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium/ carbonate mixture and thus molten nylon,6,6 is formed.
To prepare Nylon 6,6 adipic acid and hexamethylenediamine are heated at 553 K under pressure.
This reaction is a shown:
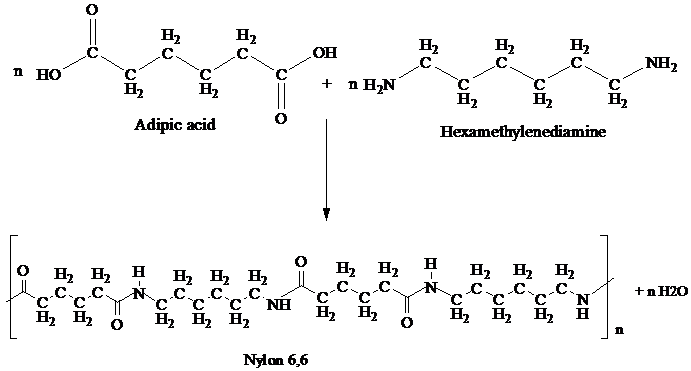
The resulting polymer is extruded into a wide range of fiber types. The fibers are drawn, or sketched in a process that increases their length and reorients the materials molecules parallel to one another to produce a strong, elastic filament.
Note:
Nylon 6,6 has long molecular chains resulting in more hydrogen bonds, creating chemical springs and making it very resilient. It is an amorphous solid so it has a large elastic property and is slightly soluble in boiling water and is very stable in nature. It is very difficult to dye, but one it is dyed it has a high colorfastness and is less susceptible to fading.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




