
Observe the following diagram and answer the questions.
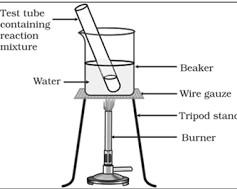
(A)What reaction is shown in the above diagram? Name the reactants and products.
(B)Write the chemical equations.
(C)What is the special characteristic of the group that is formed in this reaction?

Answer
577.5k+ views
Hint:. The reactions which generally use water baths are those which involve heating of concentrated acids or volatile organic substances such as alcohols or ethers. The above given reaction also involves acid, easily evaporated chemicals in the reaction mixture so they are kept in a water bath to be heated instead of direct heating.
Complete step by step answer:
(a) The above set up has a reaction mixture in a glass tube kept in a beaker of water (water bath) and the beaker is heated. This arrangement is made for the institutional laboratory synthesis of esters from alcohol and acid. So the reaction shown in the above diagram is an esterification reaction. The reactants of the above process are any alcohols such as ethyl alcohol ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}$/ methanol ${\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}$ and carboxylic acid such as acetic acid ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$. The products formed in the above process are ester depending upon the reacting alcohol and acid for example ethyl methyl ester.
(b) The general chemical reaction of esterification reaction is written as follows-
where, R and ${{\text{R}}^{\text{'}}}$ are the alkyl groups.
(c) The functional group formed in this reaction is ester whose general molecular formula is ${\text{R - COO}}{{\text{R}}^{\text{'}}}$. The –COO group is the ester group; it has a characteristic odor and is volatile in nature. The odor is a fruity odor which is responsible for the pleasant sweet smell of all fruits.
Note: In the process of esterification some amount of concentrated sulphuric acid is added in the reaction mixture. The acid used here can be considered as a catalyst to speed up the addition of both the reactants to give ester and water molecules.
Complete step by step answer:
(a) The above set up has a reaction mixture in a glass tube kept in a beaker of water (water bath) and the beaker is heated. This arrangement is made for the institutional laboratory synthesis of esters from alcohol and acid. So the reaction shown in the above diagram is an esterification reaction. The reactants of the above process are any alcohols such as ethyl alcohol ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}$/ methanol ${\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}$ and carboxylic acid such as acetic acid ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$. The products formed in the above process are ester depending upon the reacting alcohol and acid for example ethyl methyl ester.
(b) The general chemical reaction of esterification reaction is written as follows-
where, R and ${{\text{R}}^{\text{'}}}$ are the alkyl groups.
(c) The functional group formed in this reaction is ester whose general molecular formula is ${\text{R - COO}}{{\text{R}}^{\text{'}}}$. The –COO group is the ester group; it has a characteristic odor and is volatile in nature. The odor is a fruity odor which is responsible for the pleasant sweet smell of all fruits.
Note: In the process of esterification some amount of concentrated sulphuric acid is added in the reaction mixture. The acid used here can be considered as a catalyst to speed up the addition of both the reactants to give ester and water molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




