
Order of esterification of alcohol is
A. $3^\circ > 2^\circ > 1^\circ $
B. \[2^\circ > 3^\circ > 1^\circ \]
C. \[1^\circ > 2^\circ > 3^\circ \]
D. none of these
Answer
582.3k+ views
Hint: Formation of ester from alcohol and carboxylic acid in presence of concertation ${H_2}S{O_4}$ mirror is called esterification.
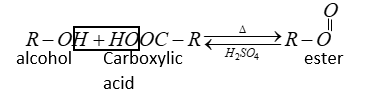
Step by step answer:
Let us explain the general reaction of esterification.
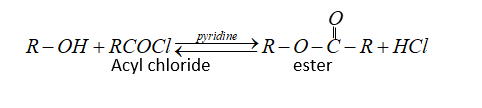
Alcohol and phenol react carboxylic acid, acid chlorides and acid anhydride to form esters.
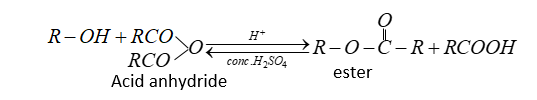
The reaction is reversible therefore water is removed by absorbing it by concentration ${H_2}S{O_4}$.
With acyl chloride,${H_4}$ is removed by pyridine.
So that reaction takes place in a forward direction.
In the esterification reaction H-atoms come from alcohol and $ - OH$ comes from carboxylic acid.
Acidic character of alcohol due to plan nature of $O - H$ bond.
An electron releasing group like $ - C{H_3}, - {C_2}{H_5}$ increases electron density on oxygen.
It decreases polarity of$O - H$bond. This decreases acid strength.
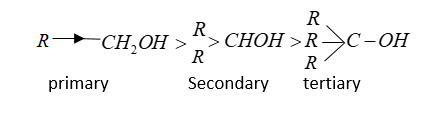
Steric hindrance (far bulkiness) increases from primary to secondary to tertiary alcohol, the order of esterification decreases. Thus the relative order of esterification of alcohol is \[1^\circ > 2^\circ > 3^\circ \].
Therefore, from the above explanation the correct option is (C) \[1^\circ > 2^\circ > 3^\circ \].
Note: In esterification carbonyl oxygen of carboxylic acid is protonated.
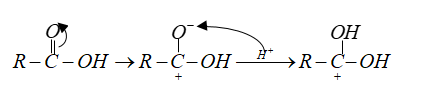
This increases the positive character of C-atoms.
The nucleophile alcohol attacks the carbonyl carbon.
Then followed by intramolecular ${H^ + }$ transfer and loss of water molecules.
In the last step deprotonation gives ester.
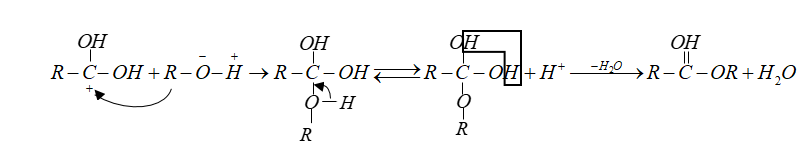
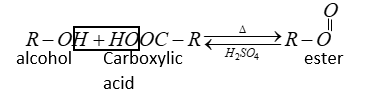
Step by step answer:
Let us explain the general reaction of esterification.
Alcohol and phenol react carboxylic acid, acid chlorides and acid anhydride to form esters.

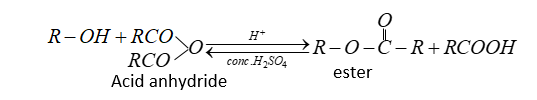
The reaction is reversible therefore water is removed by absorbing it by concentration ${H_2}S{O_4}$.
With acyl chloride,${H_4}$ is removed by pyridine.
So that reaction takes place in a forward direction.
In the esterification reaction H-atoms come from alcohol and $ - OH$ comes from carboxylic acid.
Acidic character of alcohol due to plan nature of $O - H$ bond.
An electron releasing group like $ - C{H_3}, - {C_2}{H_5}$ increases electron density on oxygen.
It decreases polarity of$O - H$bond. This decreases acid strength.

Steric hindrance (far bulkiness) increases from primary to secondary to tertiary alcohol, the order of esterification decreases. Thus the relative order of esterification of alcohol is \[1^\circ > 2^\circ > 3^\circ \].
Therefore, from the above explanation the correct option is (C) \[1^\circ > 2^\circ > 3^\circ \].
Note: In esterification carbonyl oxygen of carboxylic acid is protonated.

This increases the positive character of C-atoms.
The nucleophile alcohol attacks the carbonyl carbon.
Then followed by intramolecular ${H^ + }$ transfer and loss of water molecules.
In the last step deprotonation gives ester.
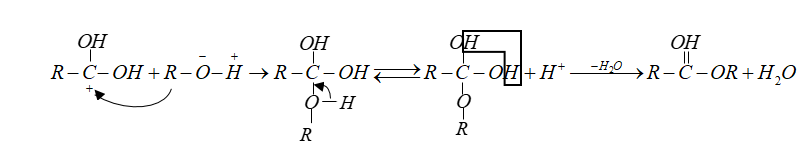
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




