
What is the order of stability for?
I 1-butene; II cis – 2 butene; III trans-2buten
(A) I < II < III
(B) I > II > III
(C) III > I > II
(D) I > II < III
Answer
558.9k+ views
Hint: Stability also depends on the symmetry of the molecule.
Stability of alkene is directly proportional to the number of $\alpha $hydrogen or in the other word we can say more the substituted alkene higher will be the stability.
Alkyl groups have $\text{+I}$(inductive effect).
Complete step by step answer:
- In 2-butene double bond present in second position while in 1-butene double bond is situated in the first position. As the number of alkyl groups around the double bond increases the stability of alkene will increase.
- 2-butene is more stable than 1-butene because of the presence of the alkyl group which has positive inductive effect. These alkyl groups repel the electron toward the double bond and stabilise the double bond. So 2-butene is more substituted then 1-butene it will be more stable.
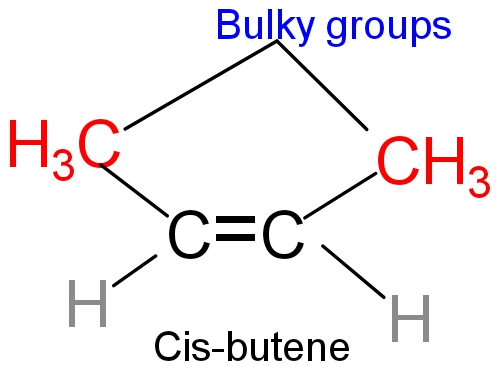
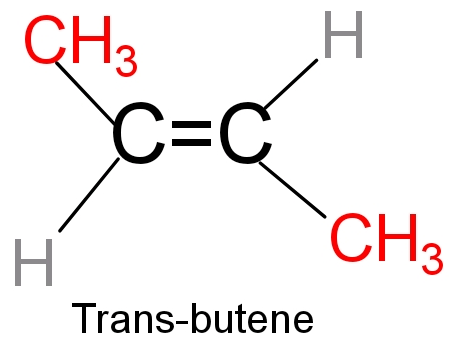
- In cis- butene two bulky groups (methyl group) are present on the same side and there is less distance between the bulky groups. So more the steric repulsion lesser will be the stability. However in trans-2 butene two bulky groups are present in the opposite of the double bond and there is more distance in between the bulky group (methyl group). So, lesser the steric repulsion higher will be stability. So trans-2butene is more stable than cis -2 butene.
So the correct answer is “A”:
Note: If a molecule is less reactive the stability of the molecule will be higher.
$\text{stability}\,\text{of}\,\text{alkene}\,\,\text{ }\!\!\propto\!\!\text{ }\dfrac{\text{No}\,\text{of}\,\text{propto}\,\text{hydrogen}}{\text{Heat}\,\text{of}\,\text{hydrogenation}}$ so if the alkene is more reactive more reaction with the hydrogen atom and it will increase the heat of hydrogenation, so lesser the stability of alkene.
Stability of alkene is directly proportional to the number of $\alpha $hydrogen or in the other word we can say more the substituted alkene higher will be the stability.
Alkyl groups have $\text{+I}$(inductive effect).
Complete step by step answer:
- In 2-butene double bond present in second position while in 1-butene double bond is situated in the first position. As the number of alkyl groups around the double bond increases the stability of alkene will increase.
- 2-butene is more stable than 1-butene because of the presence of the alkyl group which has positive inductive effect. These alkyl groups repel the electron toward the double bond and stabilise the double bond. So 2-butene is more substituted then 1-butene it will be more stable.
- In cis- butene two bulky groups (methyl group) are present on the same side and there is less distance between the bulky groups. So more the steric repulsion lesser will be the stability. However in trans-2 butene two bulky groups are present in the opposite of the double bond and there is more distance in between the bulky group (methyl group). So, lesser the steric repulsion higher will be stability. So trans-2butene is more stable than cis -2 butene.


So the correct answer is “A”:
Note: If a molecule is less reactive the stability of the molecule will be higher.
$\text{stability}\,\text{of}\,\text{alkene}\,\,\text{ }\!\!\propto\!\!\text{ }\dfrac{\text{No}\,\text{of}\,\text{propto}\,\text{hydrogen}}{\text{Heat}\,\text{of}\,\text{hydrogenation}}$ so if the alkene is more reactive more reaction with the hydrogen atom and it will increase the heat of hydrogenation, so lesser the stability of alkene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




