
Ortho-nitrophenol is less soluble in water than $p - $ and $m - $ nitrophenols because:
A: $o - $ nitrophenol shows intramolecular $H - $ bonding
B: $o - $ nitrophenol shows intermolecular $H - $ bonding
C: Melting point of $o - $ nitrophenol is lower than those of $m - $ and $p - $ isomers
D: $o - $ nitrophenol is more volatile in steam than those of $m - $ and $p - $ isomers
Answer
588k+ views
Hint: Solubility of a substance in water depends on many factors. Some of these factors are polarity and hydrogen bonding. Water is a polar molecule. As said,dissolves like, solubility of polar molecules in water is more as compared to non-polar molecules. Polar molecules are those which have some imbalance of charge in their arrangement.
Complete step by step answer:
Hydrogen bonding is the bonding that occurs between the hydrogen atom and high electronegative elements. Hydrogen makes this bonding with fluorine, oxygen and nitrogen only because these elements are highly electronegative. Electronegativity is the measure of force with which an atom can pull a shared pair of electrons. Structure of ortho-nitrophenol is:
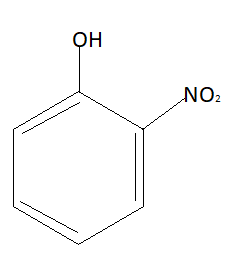
As stated above hydrogen bonding of hydrogen is possible with three elements that are nitrogen, oxygen and fluorine. In this compound $N{O_2}$ group and $OH$ group are very close to each other. Due to which intramolecular hydrogen bonding will take place in ortho-nitrophenol. Structure of para-nitrophenol is:
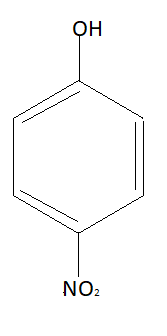
In this molecule $N{O_2}$ group and $OH$ groups are away from each other so intramolecular hydrogen bonding is not possible. Similarly structure of meta-nitrophenol is:
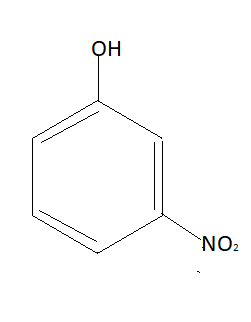
In this molecule also $N{O_2}$ group and $OH$ groups are away from each other so intramolecular hydrogen bonding is not possible. This means intramolecular hydrogen bonding is possible only in ortho-nitrophenol. Due to this hydrogen bonding hydrogen is attached to a nitrogen atom due to which it can’t be dissociated. Without being dissociated it can’t be dissolved in water. This is why ortho-nitrophenol is less soluble in water.
So, the correct answer is option A that is $o - $ nitrophenol shows intramolecular $H - $ bonding.
Note:
Term otho, meta and para are decided on the basis of position of functional groups in an atom. If the function group is attached to the carbon atom adjacent to another functional group containing carbon then it is said to be at ortho position. If the functional group is present at the carbon atom which is present at the position opposite to the carbon of another functional group then it is said to be at para position. If the functional group is in between ortho and para then it is said to be at meta position.
Complete step by step answer:
Hydrogen bonding is the bonding that occurs between the hydrogen atom and high electronegative elements. Hydrogen makes this bonding with fluorine, oxygen and nitrogen only because these elements are highly electronegative. Electronegativity is the measure of force with which an atom can pull a shared pair of electrons. Structure of ortho-nitrophenol is:

As stated above hydrogen bonding of hydrogen is possible with three elements that are nitrogen, oxygen and fluorine. In this compound $N{O_2}$ group and $OH$ group are very close to each other. Due to which intramolecular hydrogen bonding will take place in ortho-nitrophenol. Structure of para-nitrophenol is:

In this molecule $N{O_2}$ group and $OH$ groups are away from each other so intramolecular hydrogen bonding is not possible. Similarly structure of meta-nitrophenol is:

In this molecule also $N{O_2}$ group and $OH$ groups are away from each other so intramolecular hydrogen bonding is not possible. This means intramolecular hydrogen bonding is possible only in ortho-nitrophenol. Due to this hydrogen bonding hydrogen is attached to a nitrogen atom due to which it can’t be dissociated. Without being dissociated it can’t be dissolved in water. This is why ortho-nitrophenol is less soluble in water.
So, the correct answer is option A that is $o - $ nitrophenol shows intramolecular $H - $ bonding.
Note:
Term otho, meta and para are decided on the basis of position of functional groups in an atom. If the function group is attached to the carbon atom adjacent to another functional group containing carbon then it is said to be at ortho position. If the functional group is present at the carbon atom which is present at the position opposite to the carbon of another functional group then it is said to be at para position. If the functional group is in between ortho and para then it is said to be at meta position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




