
What oxidation reactions can be used to identify the configuration of sugars?
Answer
517.2k+ views
Hint: An aldose is a monosaccharide (simple sugar) with a carbon backbone chain and hydroxyl groups bound to all of the other carbon atoms, making it an aldehyde. Aldoses can be isolated from ketones, which have the carbonyl group further away from the end of the molecule.
Complete answer: An oxidising agent (also known as an oxidizer or oxidant) is a chemical species that appears to oxidise other molecules, causing them to lose electrons and thus increase their oxidation state. Halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide are also forms of oxidising agents.
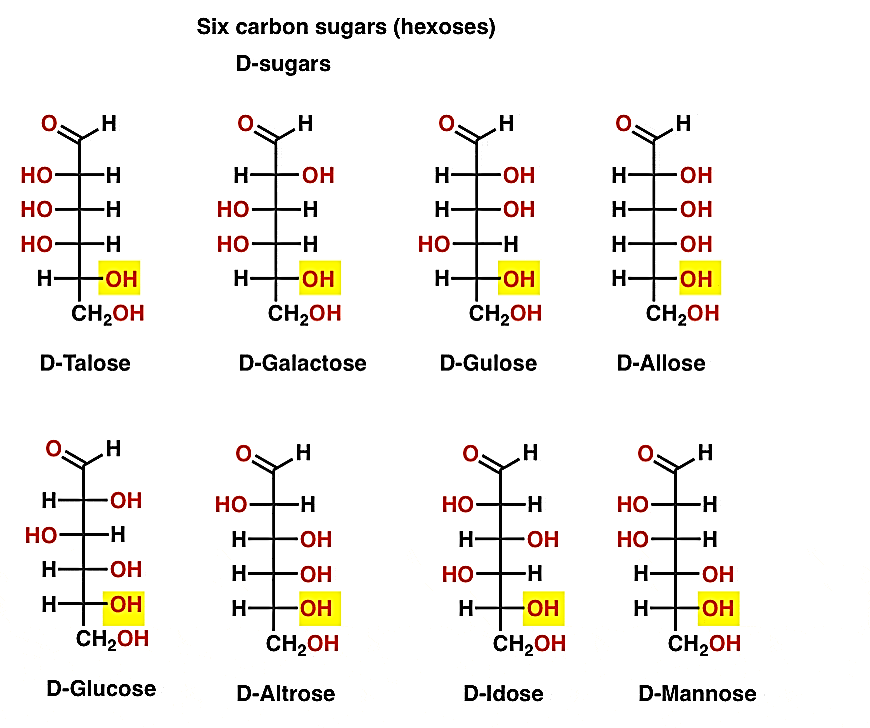
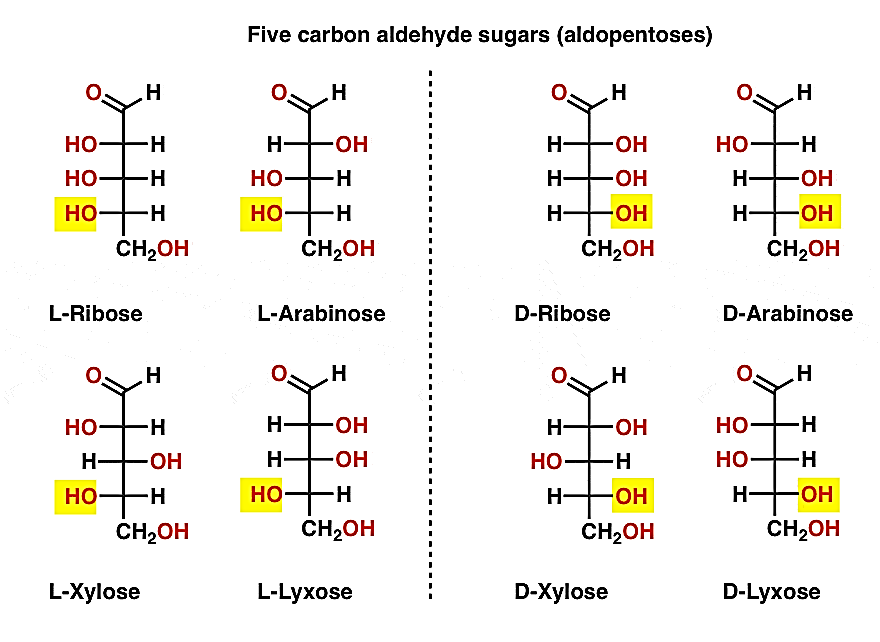
As aldose is oxidised with very mild oxidising agents such as bromine water or hypo-bromite, a monocarboxylic acid is formed (aldonic acid). As oxidising agents such as nitric acid are used, the secondary hydroxyl group in the molecule is oxidised, resulting in a dicarboxylic acid. The substance is known as an aldaric acid when the carboxylic acids are at the top. This oxidation would be helpful in locating some latent symmetry in the molecule's remaining pieces. As a result, achiral aldaric acids are formed from ribose, xylose, allose, and galactose, which are optically inactive. When (diastereomeric) aldose sugars are oxidised, identical chiral aldaric acids are produced, meaning that their configurations are similar. For example, aldaric acid obtained from arabinose and lyxose are identical.
Note:
Glucose has a D- structure while the hydroxyl groups on carbons 4 and 5 are on the right side of the fischer projection. Glucose is L-sugar while the hydroxyl groups on carbons 4 and 5 are to the left of the fischer projection.
Complete answer: An oxidising agent (also known as an oxidizer or oxidant) is a chemical species that appears to oxidise other molecules, causing them to lose electrons and thus increase their oxidation state. Halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide are also forms of oxidising agents.
As aldose is oxidised with very mild oxidising agents such as bromine water or hypo-bromite, a monocarboxylic acid is formed (aldonic acid). As oxidising agents such as nitric acid are used, the secondary hydroxyl group in the molecule is oxidised, resulting in a dicarboxylic acid. The substance is known as an aldaric acid when the carboxylic acids are at the top. This oxidation would be helpful in locating some latent symmetry in the molecule's remaining pieces. As a result, achiral aldaric acids are formed from ribose, xylose, allose, and galactose, which are optically inactive. When (diastereomeric) aldose sugars are oxidised, identical chiral aldaric acids are produced, meaning that their configurations are similar. For example, aldaric acid obtained from arabinose and lyxose are identical.


Note:
Glucose has a D- structure while the hydroxyl groups on carbons 4 and 5 are on the right side of the fischer projection. Glucose is L-sugar while the hydroxyl groups on carbons 4 and 5 are to the left of the fischer projection.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




