
Oxone is the commercial name of:
A. ${\text{KHS}}{{\text{O}}_{\text{5}}}$
B. ${\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}$
C. ${{\text{K}}_{\text{2}}}{{\text{O}}_{\text{2}}}$
D. ${{\text{K}}_{\text{2}}}{\text{O}}$
Answer
570k+ views
Hint: Oxone is the trade name of acid of sulphur. The acid has sulphur in its maximum oxidation number. The salt of the sulphur acid is known as Oxone.
Complete answer
${\text{KHS}}{{\text{O}}_{\text{5}}}$is known as potassium peroxymonosulfate. It is found as a white powder. ${\text{KHS}}{{\text{O}}_{\text{5}}}$is a salt of acid of sulphur that is ${\text{HS}}{{\text{O}}_{\text{5}}}$.${\text{HS}}{{\text{O}}_{\text{5}}}$is known as Caro’s acid.
The trade name of potassium peroxymonosulfate is Oxone. It is also known as Caroat, and MPS.
As the name indicates potassium peroxymonosulfate or Ozone has peroxy linkage.
The structure of potassium peroxymonosulfate is as follows:
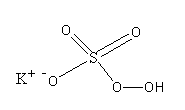
>Oxone has sulphur atoms in $ + 6$oxidation state. So, Oxone is used as an oxidising agent. It is also very corrosive. The standard reduction potential of potassium peroxymonosulfate is $1.8$ V.
>Oxone is used for disinfecting laboratory equipment.
>${\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}$is known as sodium oxide. It is used in ceramics.
>${{\text{K}}_{\text{2}}}{{\text{O}}_{\text{2}}}$is known as potassium peroxide. It is a yellow amorphous solid.
<${{\text{K}}_{\text{2}}}{\text{O}}$is known as potassium oxide. It is a pale yellow solid. It is used as N-P-K fertilizer in industries. So, Oxone is the commercial name of${\text{KHS}}{{\text{O}}_{\text{5}}}$.
Therefore, option (A) ${\text{KHS}}{{\text{O}}_{\text{5}}}$is correct
Note:Ozone and ozone are different. ${\text{KHS}}{{\text{O}}_{\text{5}}}$is known as Oxone whereas ${{\text{O}}_{\text{3}}}$is known as ozone. Ozone is an inorganic molecule. Potassium monosulfate is also different from potassium persulphate. The chemical formula of potassium persulphate is ${{\text{K}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$. Potassium persulphate is also a powerful oxidizing agent. The potassium salt Oxone is itself is a part of triple salt having chemical formula ${\text{2KHS}}{{\text{O}}_{\text{5}}}{\text{.KHS}}{{\text{O}}_{\text{4}}}{\text{.}}{{\text{K}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$.
Complete answer
${\text{KHS}}{{\text{O}}_{\text{5}}}$is known as potassium peroxymonosulfate. It is found as a white powder. ${\text{KHS}}{{\text{O}}_{\text{5}}}$is a salt of acid of sulphur that is ${\text{HS}}{{\text{O}}_{\text{5}}}$.${\text{HS}}{{\text{O}}_{\text{5}}}$is known as Caro’s acid.
The trade name of potassium peroxymonosulfate is Oxone. It is also known as Caroat, and MPS.
As the name indicates potassium peroxymonosulfate or Ozone has peroxy linkage.
The structure of potassium peroxymonosulfate is as follows:

>Oxone has sulphur atoms in $ + 6$oxidation state. So, Oxone is used as an oxidising agent. It is also very corrosive. The standard reduction potential of potassium peroxymonosulfate is $1.8$ V.
>Oxone is used for disinfecting laboratory equipment.
>${\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}$is known as sodium oxide. It is used in ceramics.
>${{\text{K}}_{\text{2}}}{{\text{O}}_{\text{2}}}$is known as potassium peroxide. It is a yellow amorphous solid.
<${{\text{K}}_{\text{2}}}{\text{O}}$is known as potassium oxide. It is a pale yellow solid. It is used as N-P-K fertilizer in industries. So, Oxone is the commercial name of${\text{KHS}}{{\text{O}}_{\text{5}}}$.
Therefore, option (A) ${\text{KHS}}{{\text{O}}_{\text{5}}}$is correct
Note:Ozone and ozone are different. ${\text{KHS}}{{\text{O}}_{\text{5}}}$is known as Oxone whereas ${{\text{O}}_{\text{3}}}$is known as ozone. Ozone is an inorganic molecule. Potassium monosulfate is also different from potassium persulphate. The chemical formula of potassium persulphate is ${{\text{K}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}$. Potassium persulphate is also a powerful oxidizing agent. The potassium salt Oxone is itself is a part of triple salt having chemical formula ${\text{2KHS}}{{\text{O}}_{\text{5}}}{\text{.KHS}}{{\text{O}}_{\text{4}}}{\text{.}}{{\text{K}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




