
Pentane has a molecular formula \[{{C}_{5}}{{H}_{12}}\]. It has:
a) 5 covalent bonds
b) 12 covalent bonds
c) 16 covalent bonds
d) 17 covalent bonds
Answer
528.2k+ views
Hint: Start this question by breaking the given compound into two parts. Try to identify the number of carbons in the compound. The first half suggests the number of carbon present in the compound and the second part suggests the number and kind of bond present between the carbon.
Complete step by step answer:
As the name suggests, pentane is made up of ‘pent’ and ‘ane’. Therefore, we can say that it has 5 carbons and it is an alkane. “An alkane is a compound that consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single.”
We have also been provided with its molecular formula - \[{{C}_{5}}{{H}_{12}}\]. The compound has 12 hydrogens.
Let us draw the structure of this compound.
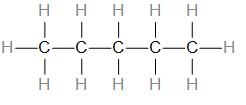
As we can see, there are –
5 C-C single bonds
14 C-H single bonds
Therefore, the answer is – option (c) – Pentane, which has a molecular formula \[{{C}_{5}}{{H}_{12}}\], it has 16 covalent bonds.
Additional Information:
Nomenclature in organic chemistry is very easy. The hydrocarbons with a single bond are called alkanes. Similarly, hydrocarbons with double bonds are called alkenes and hydrocarbons with triple bonds are called alkyne.
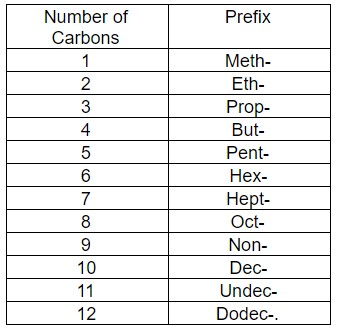
Note: The prefixes are given in the table below –
Complete step by step answer:
As the name suggests, pentane is made up of ‘pent’ and ‘ane’. Therefore, we can say that it has 5 carbons and it is an alkane. “An alkane is a compound that consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single.”
We have also been provided with its molecular formula - \[{{C}_{5}}{{H}_{12}}\]. The compound has 12 hydrogens.
Let us draw the structure of this compound.

As we can see, there are –
5 C-C single bonds
14 C-H single bonds
Therefore, the answer is – option (c) – Pentane, which has a molecular formula \[{{C}_{5}}{{H}_{12}}\], it has 16 covalent bonds.
Additional Information:
Nomenclature in organic chemistry is very easy. The hydrocarbons with a single bond are called alkanes. Similarly, hydrocarbons with double bonds are called alkenes and hydrocarbons with triple bonds are called alkyne.
Note: The prefixes are given in the table below –

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




