
Peroxide ions are present in:
A.\[{H_2}{O_2}\]
B.\[Ba{O_2}\]
C.\[O{F_2}\]
D.\[{H_2}{S_2}{O_8}\]
Answer
583.5k+ views
Hint: We know that in peroxide ions, oxygen has -1 oxidation state. So, we can calculate the oxidation of oxygen in all the four molecules to know about the presence of peroxide ions. It is known to us that an oxygen atom contributes -2 charge but peroxide has -1 charge only. Sometimes, oxide can have +2 charge as well when it is bonded to higher electronegative atoms such as fluorine.
Complete step-by-step answer:
We know that peroxide is represented as \[O_2^{2 - }\] . In this case, we have two oxygen atoms and a charge of -2 which is equally distributed among both the atoms. Thus, the charge carried by an individual oxygen atom will be \[\dfrac{{ - 2}}{2} = - 1\] . So, now we know that the oxidation number of each atom in peroxide is -1.
Let us now calculate the oxidation number of oxygen atoms in each molecule given to us.
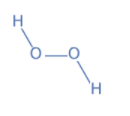
In \[{H_2}{O_2}\] , the name hydrogen peroxide itself says that it is a peroxide. In this, a hydrogen atom has +1 charge, so two hydrogens will have +2 charge. As the molecule is neutral, to balance it \[\,{O_2}\] must have -2 charge. Due to this, one oxygen atom will have -1 charge, so it has a peroxide ion.
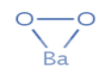
In \[Ba{O_2}\] , barium will contribute two electrons and thus it will have +2 charge on it. As the molecule is neutral, to balance it \[\,{O_2}\] must have -2 charge. Due to this, one oxygen atom will have -1 charge, so it has a peroxide ion.
In \[O{F_2}\] , two fluorine atoms are contributing -2 charge as a whole. As it is a neutral molecule, to balance it, \[\,{O_2}\] must have +2 charge. Thus, it can’t have a peroxide ion.

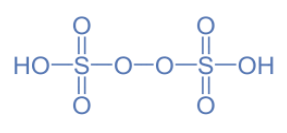
In \[{H_2}{S_2}{O_8}\] , two hydrogens will carry +2 charge and two sulphur atoms will carry +6 charge, thereby eight oxygen atoms will carry a total of -8 charge to balance the neutral molecule. This gives -1 charge to each oxygen atom. So, it definitely has a peroxide linkage and if we look at the structure, we can see two peroxide linkages in it.
Hence, the correct options are (A), (B) and (D).
Additional information:
Hydrogen peroxide which is known colloquially generally as “peroxide”, is the most toxic peroxide. It is sold at different amounts as a liquid solution. These solutions are in their own way because hydrogen peroxide is almost colourless. It is majorly used as a blanching agent and an oxidant. Nonetheless, it is also produced biochemically in the human body, due in a part to a number of oxidase enzymes. Coming in contact with organic compounds and condensed solutions is extremely harmful.
Note: Various organic and inorganic peroxides are useful as bleaching agents, initiators of polymerization reactions and also in the preparation of hydrogen peroxide and other oxygen compounds. Hydrogen peroxide is colourless and has a bitter flavour. Large amounts of gaseous hydrogen peroxide are present in the soil naturally. The hydrogen peroxide is unstable and decomposes easily to oxygen and water with release of heat.
Complete step-by-step answer:
We know that peroxide is represented as \[O_2^{2 - }\] . In this case, we have two oxygen atoms and a charge of -2 which is equally distributed among both the atoms. Thus, the charge carried by an individual oxygen atom will be \[\dfrac{{ - 2}}{2} = - 1\] . So, now we know that the oxidation number of each atom in peroxide is -1.
Let us now calculate the oxidation number of oxygen atoms in each molecule given to us.
In \[{H_2}{O_2}\] , the name hydrogen peroxide itself says that it is a peroxide. In this, a hydrogen atom has +1 charge, so two hydrogens will have +2 charge. As the molecule is neutral, to balance it \[\,{O_2}\] must have -2 charge. Due to this, one oxygen atom will have -1 charge, so it has a peroxide ion.

In \[Ba{O_2}\] , barium will contribute two electrons and thus it will have +2 charge on it. As the molecule is neutral, to balance it \[\,{O_2}\] must have -2 charge. Due to this, one oxygen atom will have -1 charge, so it has a peroxide ion.

In \[O{F_2}\] , two fluorine atoms are contributing -2 charge as a whole. As it is a neutral molecule, to balance it, \[\,{O_2}\] must have +2 charge. Thus, it can’t have a peroxide ion.

In \[{H_2}{S_2}{O_8}\] , two hydrogens will carry +2 charge and two sulphur atoms will carry +6 charge, thereby eight oxygen atoms will carry a total of -8 charge to balance the neutral molecule. This gives -1 charge to each oxygen atom. So, it definitely has a peroxide linkage and if we look at the structure, we can see two peroxide linkages in it.

Hence, the correct options are (A), (B) and (D).
Additional information:
Hydrogen peroxide which is known colloquially generally as “peroxide”, is the most toxic peroxide. It is sold at different amounts as a liquid solution. These solutions are in their own way because hydrogen peroxide is almost colourless. It is majorly used as a blanching agent and an oxidant. Nonetheless, it is also produced biochemically in the human body, due in a part to a number of oxidase enzymes. Coming in contact with organic compounds and condensed solutions is extremely harmful.
Note: Various organic and inorganic peroxides are useful as bleaching agents, initiators of polymerization reactions and also in the preparation of hydrogen peroxide and other oxygen compounds. Hydrogen peroxide is colourless and has a bitter flavour. Large amounts of gaseous hydrogen peroxide are present in the soil naturally. The hydrogen peroxide is unstable and decomposes easily to oxygen and water with release of heat.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




