
What is the $pH$ range in which phenolphthalein is colorless?
A) $0 - 8$
B) $8 - 10$
C) $10 - 12$
D) $12 - 14$
Answer
576.9k+ views
Hint: We know that Phenolphthalein is an acid-base indicator. An acid-base indicator is also called a $pH$ indicator. Indicators are usually a substance which changes its color with change in $pH$ of the solution.
Complete step by step answer:
We can draw the structure of phenolphthalein as,
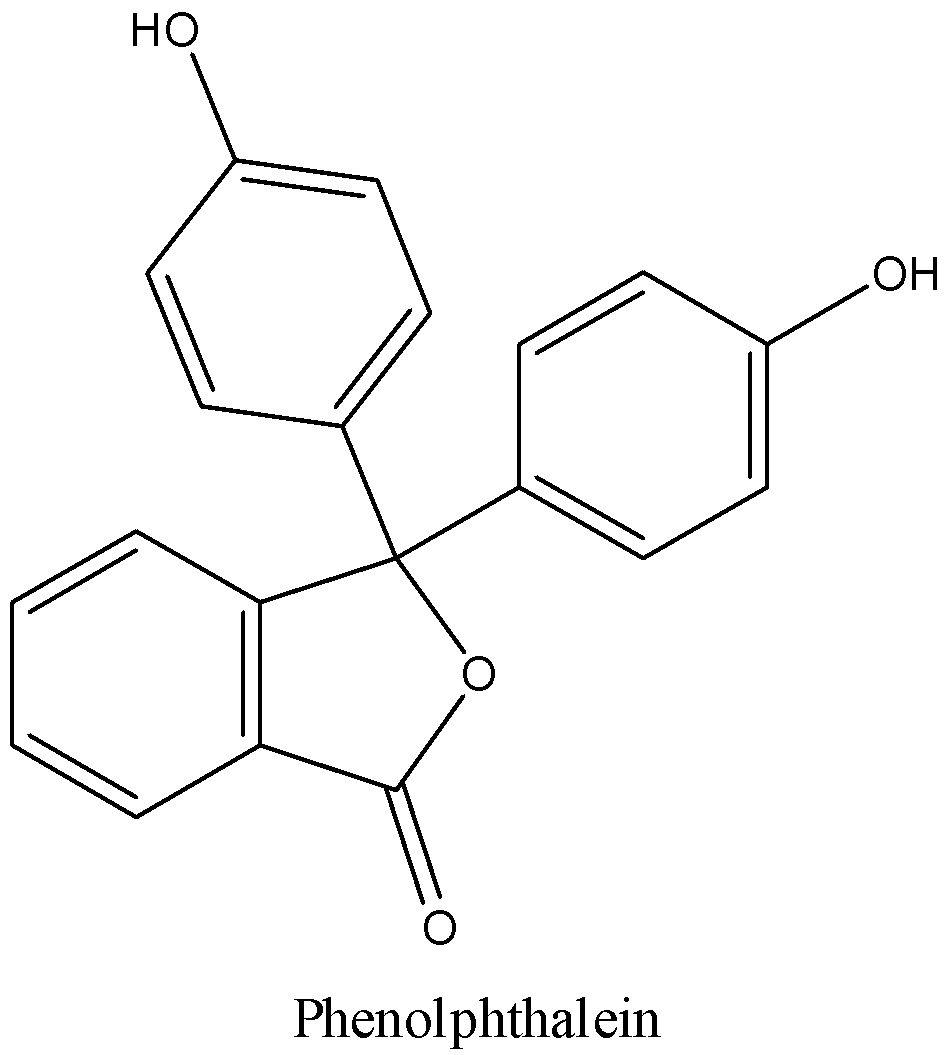
We know that the molecular formula of Phenolphthalein is ${C_{20}}{H_{14}}{O_4}$. It is a weak acid. It is a yellow crystalline solid and easily dissolves in alcohols and slightly soluble in water.
Acid-base indicators are generally weak acids or bases which dissociate when dissolved in water and form ions.
Now, consider a weak acid indicator with the formula$H{I_n}$, it dissociates in water as follows.
$H{I_n}\left( {aq} \right) + {H_2}O \rightleftharpoons {H_3}{O^ + }\left( {aq} \right) + {I_n}^ - \left( {aq} \right)$
$Acid\left( {ColorA} \right)$ $Conjugate\,base(colorB)$
The dissociation of Phenolphthalein in water is,
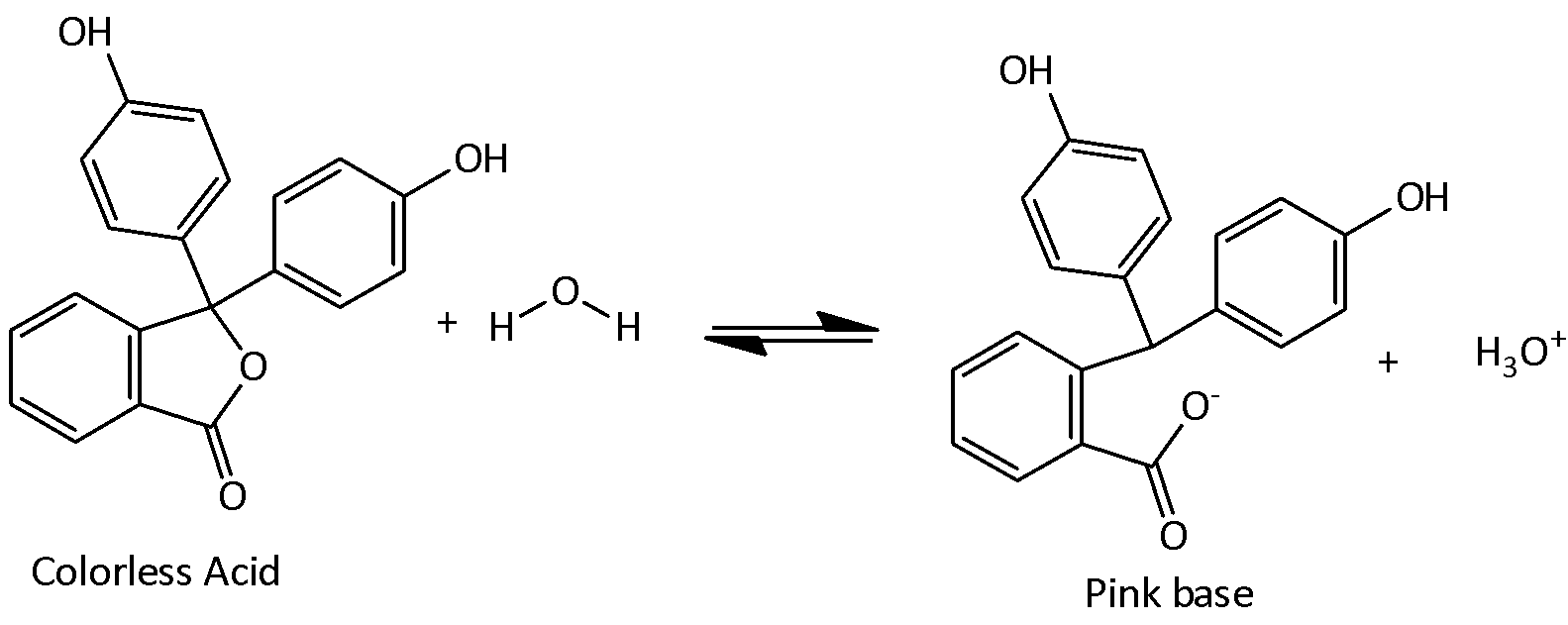
Phenolphthalein indicator used in a solution which changes its color at higher $pH$. If a weak acid is titrated against a strong base the solution changes its color when the $pH$ solution is greater than 7. Phenolphthalein remains colorless in the acidic $pH$ levels and it turns to pink at $pH = 8.2$ and continues to a bright magenta at $pH = 10.$ The color change is due to the ionization of the phenolphthalein which in turn changes the shape of the molecules.
Therefore, the correct option is A. .
Note: We know that $pH$ is the concentration of hydrogen ion in the solution.
The mathematical expression of $pH$ is,
$pH = \log \left[ {{H^ + }} \right]$
A solution which has $pH$ less than seven then is considered as acidic and a solution which has $pH$ greater than seven then it is considered as basic.
Complete step by step answer:
We can draw the structure of phenolphthalein as,

We know that the molecular formula of Phenolphthalein is ${C_{20}}{H_{14}}{O_4}$. It is a weak acid. It is a yellow crystalline solid and easily dissolves in alcohols and slightly soluble in water.
Acid-base indicators are generally weak acids or bases which dissociate when dissolved in water and form ions.
Now, consider a weak acid indicator with the formula$H{I_n}$, it dissociates in water as follows.
$H{I_n}\left( {aq} \right) + {H_2}O \rightleftharpoons {H_3}{O^ + }\left( {aq} \right) + {I_n}^ - \left( {aq} \right)$
$Acid\left( {ColorA} \right)$ $Conjugate\,base(colorB)$
The dissociation of Phenolphthalein in water is,

Phenolphthalein indicator used in a solution which changes its color at higher $pH$. If a weak acid is titrated against a strong base the solution changes its color when the $pH$ solution is greater than 7. Phenolphthalein remains colorless in the acidic $pH$ levels and it turns to pink at $pH = 8.2$ and continues to a bright magenta at $pH = 10.$ The color change is due to the ionization of the phenolphthalein which in turn changes the shape of the molecules.
Therefore, the correct option is A. .
Note: We know that $pH$ is the concentration of hydrogen ion in the solution.
The mathematical expression of $pH$ is,
$pH = \log \left[ {{H^ + }} \right]$
A solution which has $pH$ less than seven then is considered as acidic and a solution which has $pH$ greater than seven then it is considered as basic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




