
Phenol magnesium bromide reacts with methanol to give
(1) A mixture of anisole and $Mg(OH)Br$
(2) A mixture of benzene and $Mg(OMe)Br$
(3) A mixture of toluene and $Mg(OH)Br$
(4) A mixture of phenol and $Mg(Me)Br$
Answer
567.6k+ views
Hint: Recall the reaction of Grignard reagents with alcohols. General formula of Grignard reagent is $RMgX$. In the question, we are given phenol magnesium bromide which is a Grignard reagent and methanol, an alcohol.
Complete step by step solution:
We are given that phenol magnesium bromide, having chemical formula as ${C_6}{H_5}MgBr$, reacts with methanol ($C{H_3}OH$).${C_6}{H_5}MgBr$ is a Grignard reagent, having general formula as $RMgX$ where, R is any alkyl or aryl group and X is any halogen atom. Grignard reagents are good bases, that is, they abstract acidic hydrogen ions (${H^ + }$). Acidic hydrogen is any hydrogen which is attached to a highly electronegative atom like oxygen. Alcohols (general formula: $R'OH$) contain acidic hydrogen.
General reaction of Grignard reagent with alcohol can be shown as:
$R - MgX + R' - OH \to R - H + Mg(R')X$
Here, methanol ($C{H_3}OH$) has an acidic hydrogen, so phenol magnesium bromide (${C_6}{H_5}MgBr$) i.e., a Grignard reagent will attack on the acidic hydrogen of methanol. Thus, the required chemical reaction by following the above general reaction can be represented as:
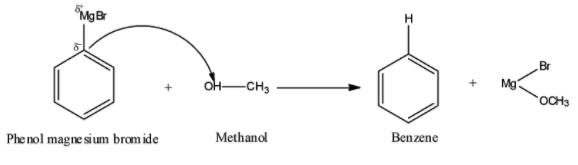
It is quite clear from the above reaction that when phenol magnesium bromide reacts with methanol, we get benzene (${C_6}{H_6}$) and $Mg(OMe)Br$ as products.
Thus, option (C) is the correct answer.
Note: Grignard reagents are generally formed by the reaction of magnesium metal with alkyl or aryl halides. It should be noted that Grignard reagents, besides being good bases, are also extremely good nucleophiles. As nucleophiles, they react with electrophiles such as carbonyl compounds (aldehydes, alcohols, esters, epoxides etc.). When Grignard reagent reacts with aldehydes and ketones, we get alcohols whereas when Grignard reagent reacts with alcohol, we get the corresponding alkane as a product.
Complete step by step solution:
We are given that phenol magnesium bromide, having chemical formula as ${C_6}{H_5}MgBr$, reacts with methanol ($C{H_3}OH$).${C_6}{H_5}MgBr$ is a Grignard reagent, having general formula as $RMgX$ where, R is any alkyl or aryl group and X is any halogen atom. Grignard reagents are good bases, that is, they abstract acidic hydrogen ions (${H^ + }$). Acidic hydrogen is any hydrogen which is attached to a highly electronegative atom like oxygen. Alcohols (general formula: $R'OH$) contain acidic hydrogen.
General reaction of Grignard reagent with alcohol can be shown as:
$R - MgX + R' - OH \to R - H + Mg(R')X$
Here, methanol ($C{H_3}OH$) has an acidic hydrogen, so phenol magnesium bromide (${C_6}{H_5}MgBr$) i.e., a Grignard reagent will attack on the acidic hydrogen of methanol. Thus, the required chemical reaction by following the above general reaction can be represented as:

It is quite clear from the above reaction that when phenol magnesium bromide reacts with methanol, we get benzene (${C_6}{H_6}$) and $Mg(OMe)Br$ as products.
Thus, option (C) is the correct answer.
Note: Grignard reagents are generally formed by the reaction of magnesium metal with alkyl or aryl halides. It should be noted that Grignard reagents, besides being good bases, are also extremely good nucleophiles. As nucleophiles, they react with electrophiles such as carbonyl compounds (aldehydes, alcohols, esters, epoxides etc.). When Grignard reagent reacts with aldehydes and ketones, we get alcohols whereas when Grignard reagent reacts with alcohol, we get the corresponding alkane as a product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




